

This activity is aimed at GCSE students. The material here is part of the WJEC curriculum for Chemistry and Double Science
https://commons.wikimedia.org/wiki/File:Periodic_Table_Armtuk3.svg
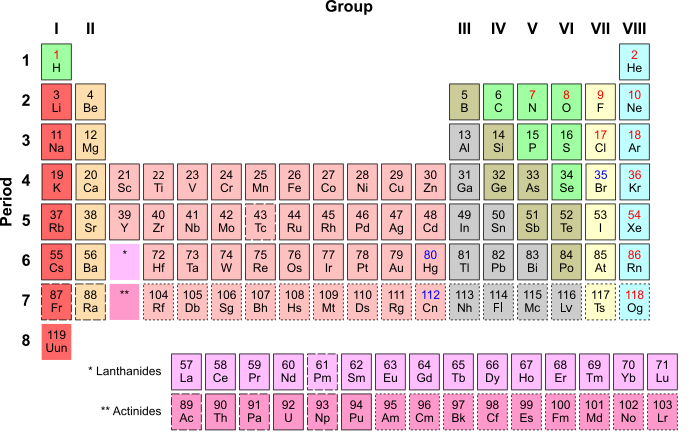
Look at carbon's position on the Periodic Table of Elements shown above.
What does its position on the table tell us about the properties and structure of Carbon?
How many electrons does carbon have?
How many shells does carbon have?
How many electrons on the outer shell?
What is covalent bonding?
Carbon has four electrons in its outer shell meaning it can 'share' an electron with up to four other atoms.
This allows carbon to form giant covalent structures.
The first giant covalent structure of carbon we shall look at, is graphite.

https://commons.wikimedia.org/wiki/File:Graphite-233436.jpg
Graphite is the material used as 'lead' in pencils.
With this is mind, what properties does graphite have?
Let us zoom in closer to see how the carbon atoms are bonded together.
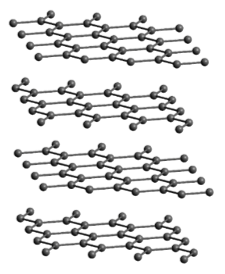
Each carbon atom is attached to three others in a 2-dimensional layer (called graphene). The graphite is made up of lots of these layers.
With this information, try to work out the following:
Why does graphite have a high melting point?
Why is graphite soft and easily broken?
Why can graphite conduct electricity?
Covalent bonds are strong
There are only weak bonds between layers
The spare fourth electron from each atom moves freely between the layers
Diamonds are another form of carbon in a giant covalent structure.

https://www.flickr.com/photos/jurvetson/156830367
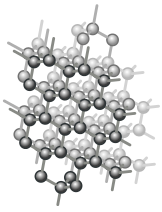
The below image shows the structure of carbon atoms within diamond.
https://commons.wikimedia.org/wiki/File:Carbon_allotropes.svg
Compare this to the structure of graphite to answer the following questions.
Why does diamond have a higher melting point (approx. 4027 ℃) than graphite (approx. 3600 ℃)?
Why is diamond a harder material than graphite?
Can diamond conduct electricity? Explain your answer.
Here is the link to the answer sheet, please only use this once you have attempted the questions yourself.
Here is a link to the WJEC Question Bank regarding past exam questions related to this worksheet.