Light quality as a developmental cue in plants
Plants respond to light
(i)
Quantity (expressed in terms of fluence rate
photon dose mmol sec-1 m2)
(ii)
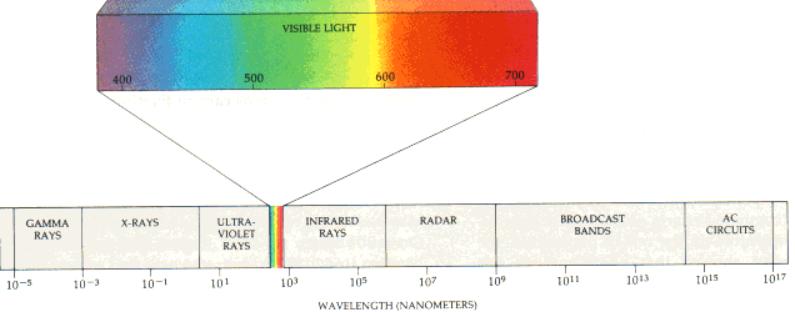
Quality (wavelengths)
(iii)
Direction – phototropism.
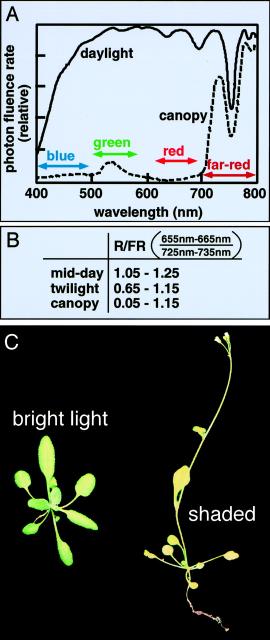
Light
quality changes during the day and if plants are shaded.
Light
quality variation in due the increased atmospheric “thickness” at dawn and
sunset tending to allow only longer light wavelengths to penetrate to the earth
surface.
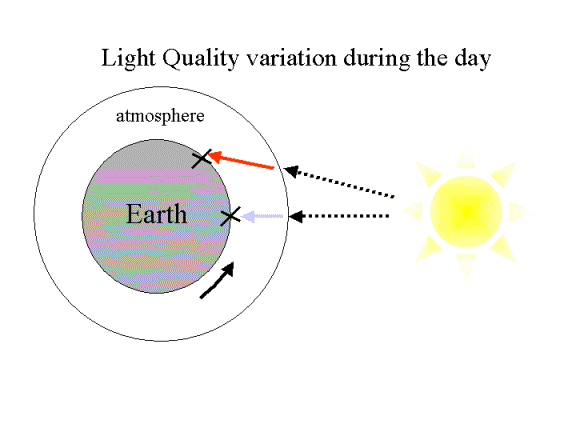
Hence,
at ~noon Blue light dominates whilst either earlier or later Red/Far-Red
wavelengths predominate i.e. light quality can act as a clock.
Day
length varies over the year and this can also be detected using light quality
detection systems, i.e. plants have a calendar.
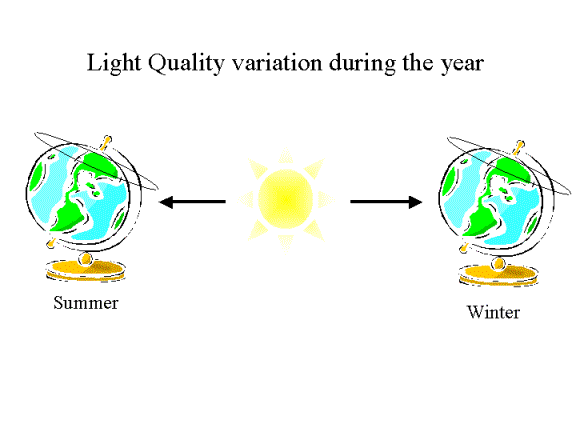
Hence
plants have evolved the ability to detect .......
(i)
UV and Blue light – cryptochromes and
phototropins
(ii)
Red light – phytochromes
Blue light detection
Cryptochromes
Blue (390–500 nm) and ultraviolet-A (UV-A; 320–390
nm) light elicit a variety of physiological responses in plants. Of these,
Most maximize photosynthetic potential in weak light
and prevent damage to the photosynthetic apparatus in excess light.
·
Phototropism
— bending towards the light — is one of the best known plant tropic responses,
·
Light-induced
opening of stomata (cooling of leaf)
·
chloroplast
migration in response to changes in light intensity (protection)
· solar tracking by leaves of
certain plant species
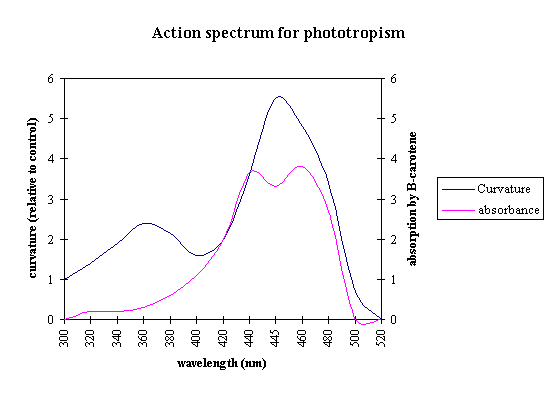
Action spectrum typically observed for
phototropin-mediated responses. Notice the presence of a major peak at 450 nm,
a shoulder at 425 nm and a minor peak at 470 nm in the blue region of the
spectrum. This fine structure is not observed in the broad absorption band at
365 nm in the ultraviolet region of the spectrum.
Despite
being reported by Darwin and others, over a century ago to be specifically
under the control of blue light, the photoreceptors have only just become
known.
Blue-light
photoreceptor from Arabidopsis, are named CRY1 and CRY2 (cyptochrome 1 and 2).
This
photoreceptor is a flavoprotein that mediates numerous
blue-light-dependent responses.
Arabidopsis, mutant
plants lacking both the CRY1 and the CRY2 blue-light photoreceptors are
deficient in the phototropic response.
Transgenic
Arabidopsis plants overexpressing CRY1 or CRY2 show enhanced phototropic
curvature.
Phototropins
Phototropins
1 (phot1) and 2 (phot2) - the most recently characterized blue-light receptors
in plants, have spectral properties.
Both
phot1 and phot2 mediate not only phototropism, after which they were named, but
also blue-light-induced chloroplast migration and blue-light-induced stomatal
opening In addition, the rapid inhibition of stem growth by blue light is
probably mediated by phot1.
phot1
also plays a role in blue-light-mediated calcium uptake and might have a minor
role in blue-light-induced membrane depolarization.
Arabidopsis mutants with an impaired
phototropic response (designated nph for non-phototropic hypocotyl) led
to the cloning and characterization of the first phototropin gene.
Four
loci identified (NPH1–NPH4)
NPH1
protein is a classic serine/threonine kinase.
The
N-terminal half of the protein contains two repeated domains of ~100 amino
acids with ~40% amino acid sequence identity. These domains are regulated by
light, oxygen or voltage and are given the acronym LOV. LOV domains constitute
a subset of the PAS-domain superfamily, which is known to mediate both ligand
binding and protein–protein interactions.
Crucially, the LOV domains are flavine mononucleotide (FMN)
binding domains
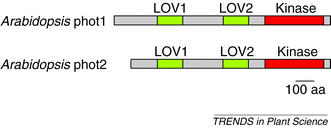
Protein
structures of the Arabidopsis blue-light receptors, phot1 and phot2 (996
and 915 amino acids, respectively). Light, oxygen or voltage (LOV) domains are
shown in green. The kinase domains, which catalyse the phosphorylation of proteins
on specific amino acid residues (threonine and serine in this case), are shown
in red.
When
irradiating isolated LOV domains with blue light, they undergo a complex
spectral change. This They fail to absorb
in the blue region of the spectrum though a new peak appears near 390 nm. These
light-induced absorbance changes result in the formation of isosbestic points at 330 nm, 375 nm and 410
nm ( see below, arrows). The observed light-induced spectral changes are fully
reversible in darkness.
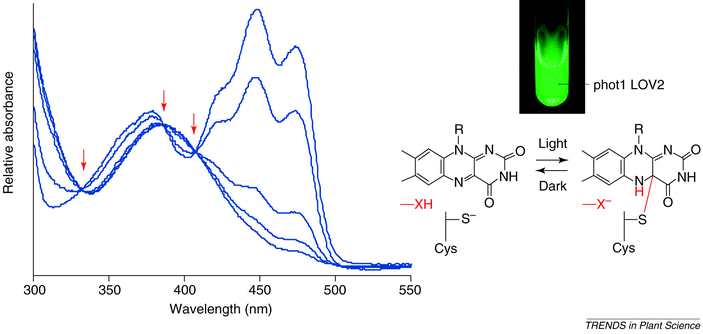
These changes result from the formation of an adduct between a cysteine residue and the C(4a) carbon of the flavin.
Mutation of a highly conserved cysteine in LOV1 and
LOV2 to alanine or serine completely abolishes their photochemical reactivity.
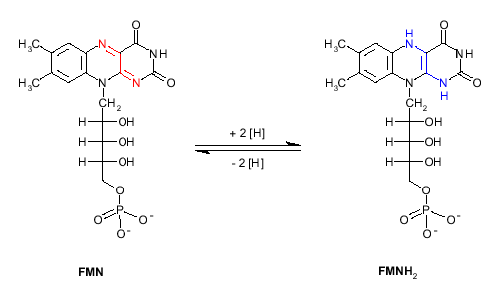
It is likely that blue-light mediated excitation of
FMN to form FMNH2 is a key
step in the formation of this cysteine adduct.
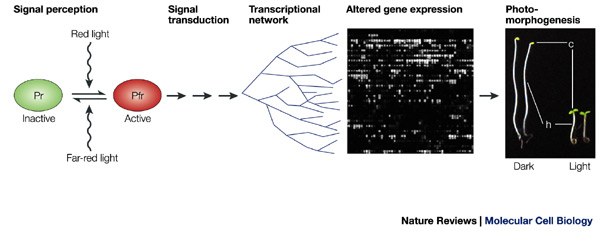
Phytochromes
First discovered in the 1950s when it was found
that a brief pulse with red-light
(i)
Initiated seed germination in dark
(ii)
De-etiolation
(iii)
Inhibition of leaf elongation
(iv)
Regulation of flowering
In fact - the red/ far red ratio (R:FR
660/730nm) is the vital factor in phytochrome mediated events.
Found to exist in two forms “Pr and Pfr” with
differing spectral properties.
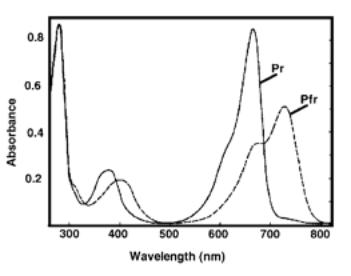
Note that Pr has a peak absorbance at 660nm
whilst Pfr absorbs maximally at 730nm.
It was found that these represented interchangeable
forms of the same protein.
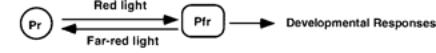
The Pr is the inactive form which is converted to the Pfr form bt RED (660nm) light. The Pfr form is active but is converted to the Pfr form by far-red light (730nm).
Evidence – Consecutive 5min treatments

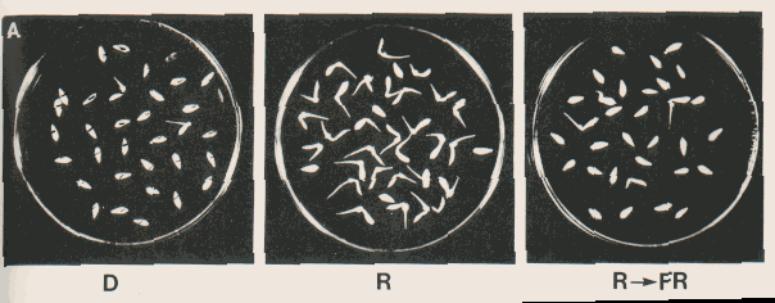
Five phytochromes have been characterised in the
model plant Arabidopsis thaliana.
Two classes /types of phytochrome
Type I (Gene PhyA)
· Accumulates to high levels in etiolated seedlings
· Unstable in light – falling to 1 to 2% original levels in the light.
· Degraded using the ubiquitin – ligase pathway
Type II (Genes PhyB, C, D and E).
· Accumulated to low concentrations in green leaves
· Stable in light
How much light?
Variable phytochrome responses.
Types of phytochrome response –
1. Very low fluence (VLF).
·
Used in soil surface detection
·
0.001mmol threshold
·
Not photoreversible
·
PhyA mediated
2. Low fluence (LF).
· Used in seed germination, shade
avoidance and stem elongation.
· 1mmol threshold
· Photoreversible
· PhyB (Type II)- mediated.
3. High irradiance (HIR).
· Used in Photoperiod detection
· 100 mmol
threshold
· Not photoreversible
· Both phyB and phyA are involved.
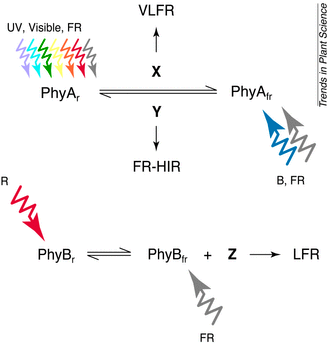
Different modes of photoperception by phyto-chrome A
(PhyA) and phytochrome B (PhyB). Phytochrome partners are represented by X, Y
and Z, which are pathway-specific or shared in the pathways. Abbreviations: B,
blue light; FR, far-red light; FR-HIR, far-red-light-mediated high irradiance
response; LFR, low fluence response; PhyAfr, PhyA in
far-red-light-absorbing form; PhyAr, PhyA in red-light-absorbing
form; PhyBfr, PhyB in far-red-light-absorbing form; PhyBr,
PhyB in red-light-absorbing form; R, red light; VLFR, very low fluence response;
UV, ultraviolet light.
Phytochrome induced
gene expression.
Microarray analysis has found
Photosynthetic genes encoding chloroplast proteins e.g.
Rubisco
(Ribulose 1,5-bisphosphate
carboxylase – small subunit)
Developmental genes e.g. chalcone synthase –
which is
involved in anthocyanin synthesis
(amongst
others).
Glycolysis and TCA cycle
Suppresses
Cell wall –loosening enzymes
Water – channel forming proteins (aquaporins).
Molecular properties of phytochrome.
Phytochrome is an apoprotein + bilitriene
chromophore → hytochromobilin.
The apoprotein is a homodimer
(120→127kDa)
Phy A- E share 50%/80% identity
Key features
The chromophore – phytochromobin (PfB) is attached in an invariant cysteine. This
is
identical in each phytochrome – ie only the apoprotein changes.
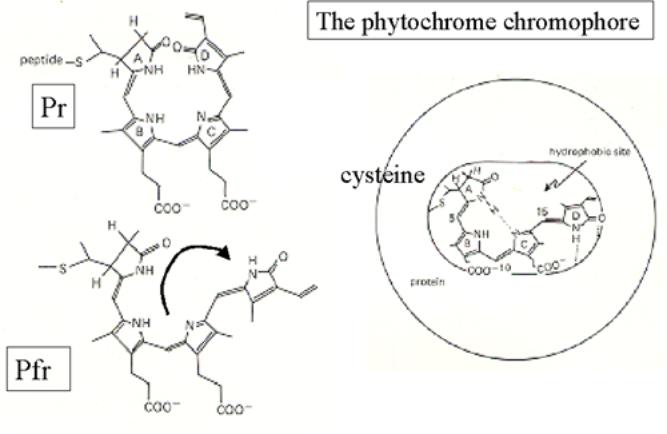
The spectral shift comes about due to the
geometric isomerisation –
C15 double bond between pyrrole rings C and D.
- of a linear tetrapyrrole ring within the
chromophore.
Note : PhyA remains active in far-red light – even
though shows the same isomeration.
Thus the PhyA Pfr form is active!
Chromophore binding region (CBD)
PAS domains – A B exhibit homology to similar
domains in mammalian systems. Allow intra-molecular interactions within the
phytochrome molecule.
HERD1/2 – histidine kinase related domains BUT
actually appears to be a serine threonine kinase.
Different phytochromes (PhyA and B)
phosphorylate different substrates.
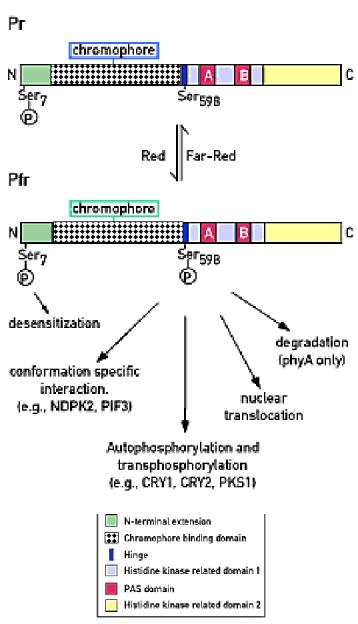
Downstream signaling from phytochrome.
Evidence
form biochemical analyses.
Cytoplasmic signaling
(i)
The switch to Pfr triggers a
heterotimeric GTP binding protein
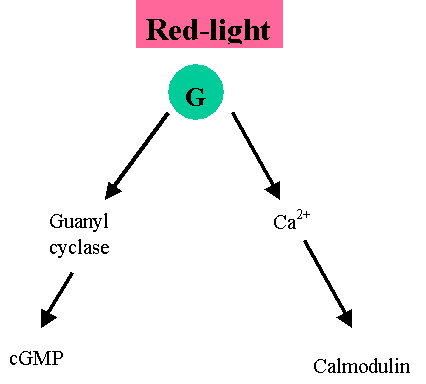
cGMP lead to the production of “anthocyanins” and
Calcium initiates chloroplast development.
However, what proteins calmodulin and cGMP interact with is still obscure.
The elucidatory role of Arabidopsis
mutants
Some terminology –
Photomorphogenesis – basically light induced
developmental changes but usually refers to de-etiolation.
Skotomorphogenesis – dark associated
development – etiolation.
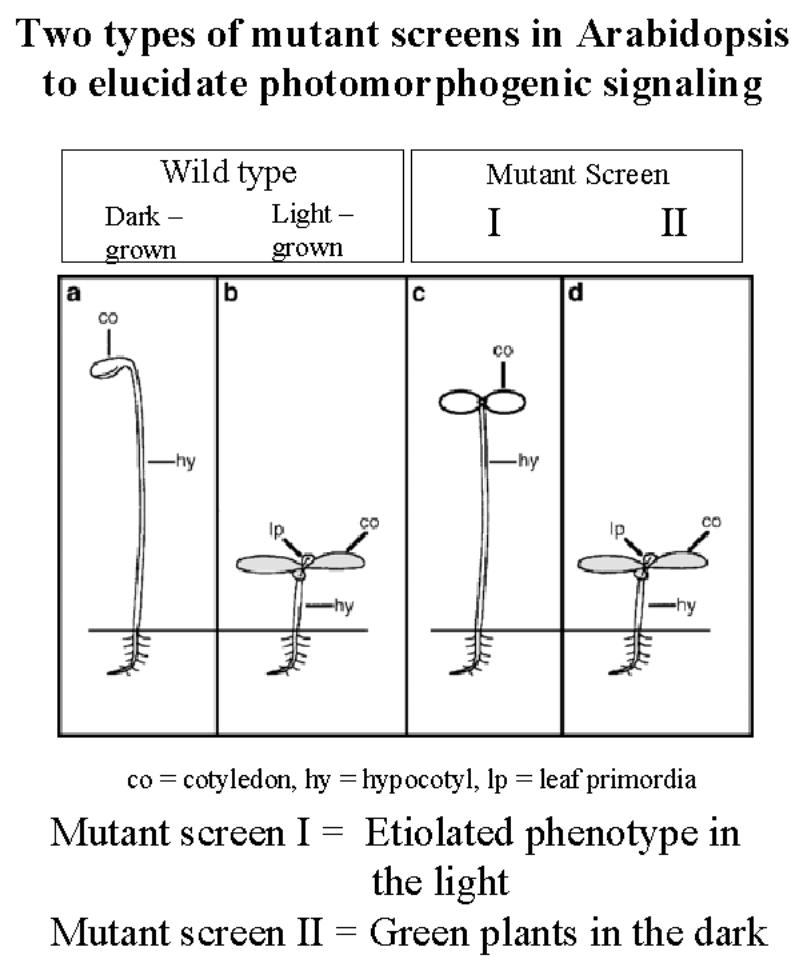
(i)
Screen to for light grown plants which look like
etiolated plants.
These
isolated chromophore biosynthesis genes –
hy1, hy 2, cry1 and cry2.
As
well as all five photoreceptors - phyA→E which are mutations in the apoprotein.
But
NOTE -NO DOWNSTREAM MUTANTS
IN
RED-LIGHT.
However,
PhyA far-red mutants have been
isolated (see below). Demonstrates that this form is active.
(ii)
Screen for dark grown plants which look like
light grown plants-
Aiming
to isolate elements in a “suppressive complex”.
COP
series → “Constitutive photomorphogenesis”
DET
De-etiolation mutants
FUS Fusca
Comprise
the COP9 signalosome (CSN) with eight sub-units.
The
CSN proteins bind to Hy5 – a transcription factor involved in activating light
dependent gene expression.
A recent
model has suggested the CSN acts by targeting Hy5 for degradation to the 26S proteosome.
This
is localised to the nucleus in the dark. In the light
Hy5
is “released”, the CSN complex is translocated to the cytoplasm - probably by
interaction with the cytoplasm - and eventually degraded.
COP9
shows some similarity to the lid complex of the 26S proteosome.
Downsteam signalling 1
: Phytochrome as a kinase
Phytochrome – an atypical – light regulated-
serine protein kinase.
Phosphorylates and autophosphorylates.
Substrates
(i)
Phytochromes proteins .
PhyA
has a higher autophosphorylation activity in the Pfr form.
(ii)
CPR5 – translocation to nucleus
(iii)
G-box BF – translocation
(iv)
Nucleotide diphosphate kinase 2 (NDPK2). NDP →NTP.
(v)
PKS1 –
Nucleotide diphosphate kinase 2 (ndpk2)
Nucleotide diphosphate kinase(NDPK) is a
multifunctional protein.
The primary role of NDPK is synthesising (d)
NTP fropm (d) NDP.
Many biological phenomena are associated with
NDPK, however these cannot be explained by NDP kinase activity.
In Drosphila , the mutation in the ndpk
gene (awd) results in
abnormal wing development.
In humans, ndpk has been identified as a tumour
suppressor, nm23.
Recently, a protein kinase function has been
detected. NDPK can phosphorylate both serine/threonine and histidine/asparate
residues.
Other functions, e.g. activating G-proteins,
have also been suggested.
PKS1
PKS1 – phytochrome kinase substrate – 439aa
Identified via two hybrid screens using the
PhyA C-terminus as a “bait”.
PKS1 seems to bind both Pr and Pfr forms.
But it is phosphorylated in a light dependent
manner.
Anti-sense did not yield any phenotype – are
other genes compensating? – functional redundancy?
However, if PKS1 was over-expressed – lines had
elongated hypocotyls -
PKS1 is a PhyA(?)-mediated negative regulator
of PhyB. PKS1-GFP fusions remain in the cytoplasm so PKS1 may act by inhibiting
PhyB action in the cytoplasm or prevent translocation to the nucleus.
Downstream signaling 2 : Change in
Cellular localisation
(i)
Transcription factor mobilisation.
Some transcription factors appear to be sequested
in the cytoplasm and migrate to the nucleus in response to red-light.
·
G-box binding (GACGTx)
transcription factors which have been found to bind light- regulated promoters.
·
CPRF2 – common promoter-binding transcription
factor family.
This process appears to involve transcription factor phosphorylation – presumably by phytochrome serine threonine kinases.
Some phytochrome interactions with AUX/IAA proteins. These are induced by auxin – hormones which modulate plant development.
(ii)
Mobilisation of phytochrome itself
In the Pr form, phytochrome proteins are found in ordered arrays along the plasma membrane.
PhyB-GFP showed nuclear localization in red-light
which reversed in Far-red.
PhyA-GFP also exhibited red induced nuclear
targeting but this could not be reversed by cFR but my pulsed FR.
Note – Differences in the number and size of
Phy
A and B complexes.
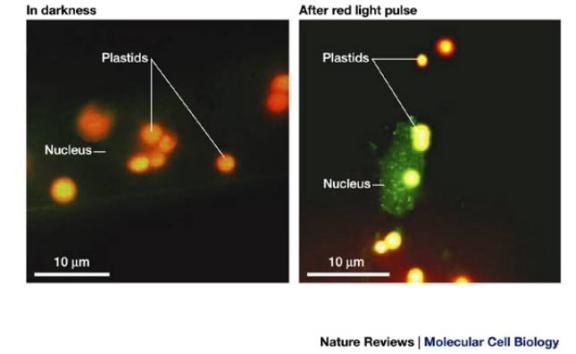
Light-induced nuclear translocation of phyA. A phyA GFP
fusion in transgenic seedlings is uniformly distributed throughout the
cytoplasm (and therefore invisible!) in darkness. A five-minute pulse of red
light induced a rapid translocation of phyA-GFP into the nucleus.
Mutation of the phy A signalling
pathway.
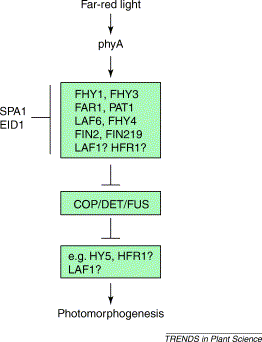
A simplified genetic model
for the phyA-mediated signalling pathway. The genes encoding all these components
excpet FIN2 andFHY4 have been cloned.
A series of mutants have been isolated which
show less sensitivity to continuous far-red light.
Of particular note
LAF1 (long after far-red light) myb-type
transcription factors
LAF6 nuclear-localised ATP binding cassette
involved in communications between
plastids and the nucleus.
Suppressors.
Mutants show enhanced phyA-specific responses
SPA1 “suppressor of phyA”
EID1
Both are involved in targeting proteins to the
26S proteosome.
Nuclear Events: transcriptional
activation
PIF3
PIF3 is a nuclear basic helix-loop-helix (H-L-H)
transcription factors which binds as a dimer.
The binding site was determined and found to be
a G-box motif.
Binds to DNA in the absence of phytohrome
proteins
But will interacts with the PAS domain of PhyA
and B only upon conversion to
the Pfr form.
This interaction was also
photo-reversible.
It has been proposed that PIF3 in conjunction
with phytochrome will active light responsive gene expression.
PIF3 will not activate all light- responsive genes with G-boxes.
Anti-sense PIF3 only suppressed
CIRCADIAN
CLOCK ASSOCIATED 1 (CCA1)
LATE
ELONGATED HYPOCOTYLS (LHY)
Both myb-like transcription factors are
involved in regulating the circadian rhythms.
The hy5 mutant – (involved in
detiolation) did not display any change in CCA1 and LHY
Thus, PIF3 may be detecting very low (night- phyA) and low (day –phyB) fluence reactions – PHOTOPERIOD.
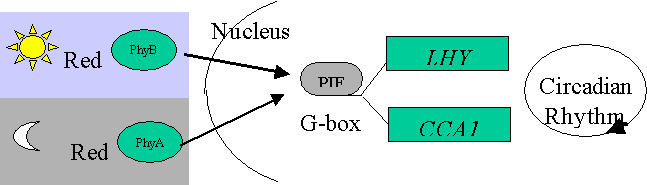
A prediction of this model is that phyA and phyB
can act antagonistically.
PhyA over-expression – (therefore present in
daylight) suppresses day-length perception.
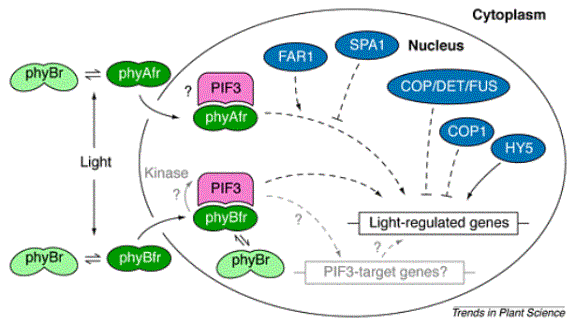
A model
for phytochrome signal transduction built around selected cloned intermediates.
The activation of light-regulated genes is mediated by a complex of Arabidopsis
nuclear phytochrome B (phyB) in the phytochrome far-red form (phyfr) and
phytochrome interacting factor3 (PIF3). Phytochrome A and phyB might operate by
similar mechanisms. The light-regulated genes are under additional control by
phyA-specific (FAR1, SPA1) or general regulators (HY5, COP1 and other
COP/DET/FUS proteins). COP1 and HY5 are known to interact . Whether
PIF3 operates via genes that are independent of COP/DET/FUS and HY5 proteins is
not known. Unbroken arrows denote physical movement or chemical modification.
Broken arrows indicate genetic interactions. Elements of the model without
experimental support are depicted in gray.
Can be developed into a model for day-length detection.
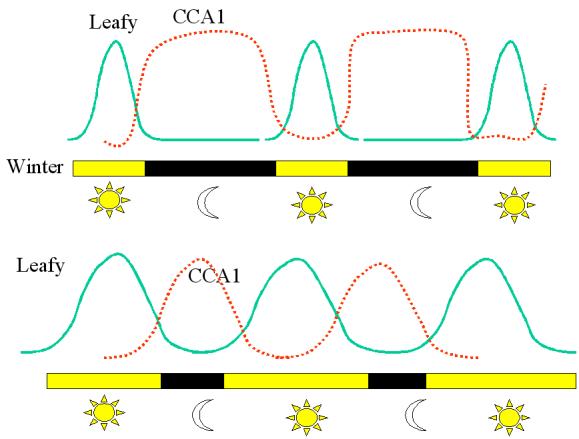
SUMMARY
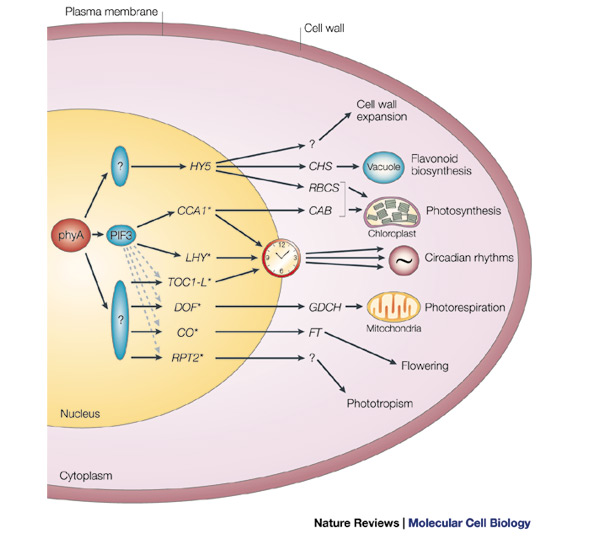
A
simplified model of phyA-regulated transcriptional network.
It is proposed that a master set of rapidly responding
transcription-factor genes (HY5–RPT2 here, with *
representing those genes with a G-box motif in their promoter) are primary
targets of phyA signalling through constitutively present transcriptional regulators
(PIF3 and other potential factors that are indicated by question marks) that
are direct recipients of incoming phyA signals. This master set of genes is
proposed to encode transcriptional regulators that control one or more main
branches of the transcriptional network that drives the various facets of
photomorphogenesis, such as cell-wall expansion and chloroplast biogenesis.
Dashed arrows indicate potential
regulation of G-box-containing genes by
·
CAB, CHLOROPHYLL A/B-BINDING
PROTEIN;
·
CCA1, CIRCADIAN CLOCK-ASSOCIATED
PROTEIN 1;
·
CHS, CHALCONE SYNTHASE;
·
CO, CONSTANS;
·
DOF, H-PROTEIN PROMOTER-BINDING
FACTOR-2A;
·
GDCH, H-PROTEIN SUBUNIT OF
GLYCINE DECARBOXYLASE;
·
FT, FLOWERING
LOCUS T;
·
HY5, LONG HYPOCOTYL 5;
·
LHY, LATE ELONGATED HYPOCOTYL;
·
PIF3, PHYTOCHROME-INTERACTING FACTOR 3;
·
RBCS, RIBULOSE
BIPHOSPHATE CARBOXYLASE SMALL SUBUNIT;
·
RPT2, ROOT
PHOTOTROPISM 2;
·
TOC1-L, TIMING OF CAB 1
EXPRESSION-LIKE