PLANT CELL TISSUE CULTURE
Organogenesis
Relies on the ability to most cells to "dedifferentiate"
and revert to a being "stem-cell".
i.e. actively dividing with no clear variation
in cell type.
In plants, this is referred to as the formation of MERISTEMS
and in culture is typified by the formation of callus tissue.
Various explants or suspension cell cultures can be used to
induce meristemic tissue when cultures with are treated with
HIGH AUXIN AND CYTOKININ LEVELS.
By changing the in
hormones in the culture medium it is possible to induce callus cultures to
re-differentiate to form viable plants.
Plant Meristems -
To some degree equivalent to mammalian “stem
cells”
Plant cells are truly totipotent. (toton = latin
= entire)
In mammals only the fertilised egg is truly
totipotent.
Really pluripotent (pluris = latin =
many several).
In animals the mature embryo is a miniature
variant of the adult animal.
Remember that meristems are active sites of hormone, particularly auxin, production.
Embryonic embryo development
Zygotic Embryo Development - Covers development from the time of fertilisation until
seed formation occurs.
The major roles for the
embryogenic process are:
1.
Establish
the basic body plan.
2.
Set aside meristematic tissue for postembryonic
elaboration of structure (leaves, roots flowers, etc.)
3.
Establish an
accessible food reserve for the germinating embryo until it becomes
autotrophic.
Following fertilization, the embryo
develops within an embryo sac which is surrounded by the maternal diploid
tissue of the ovule.
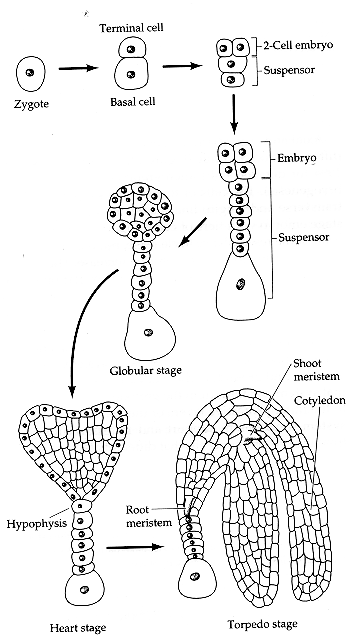
Stage 1
Axis – apical / dorsal produced early in
development.
Evidence for this polar movement of auxin.
How is this established? From maternal cells?
Apical Cell – produces embryo
Basal Cell – produces filamentous suspensor, 6-
8 cells long.
The suspensor connects embryo to maternal
tissue.
- Orientates the absorptive surface of the embryo
toward its food source
- Serves as a nutrient conduit for the developing
embryo.
Stage 2
Division lead to the formation of a 16-cell
ball ….Pro-embryo
Unknown signals from the Proembryo establishes
the HYPOPHYSIS
This is the uppermost cell of the suspensor…
The hypophysis gives rise to the quiescent
organising centre of the root meristem.
First defined shoot meristem marker is WUSCHEL
(WUS) produced at the centre of the 16 cell pro-embryo.
Central cells divide radially to form to “heart shape”
Stage 3
Heart stage embryo ~250 cells
1.
Radial
patterning emerges in the globular stage as the three tissue systems (dermal,
vascular, and ground) of the plant are initiated.
2.
The dermal system will form
from the protoderm and contribute to the outer protective layers of the plant.
3.
The
vascular system
functions in support and transport and arises from the procambium cells that
differentiate in the centre of the globular embryo.
4. Ground tissue forms from the ground
meristem and surrounds the developing vascular tissue; however, the ground and
vascular systems form independently.
Position
rather than clonal origin appears to be the critical factor in embryo pattern
formation
Suggest
cell-cell communication
E.g.
microsurgery experiments on somatic carrot embryos demonstrate that isolated
pieces of embryo can often replace the missing complement of parts.
Shoot Meristem.
Meristem divide to form two types of cells.
(a)
stem cells remaining at the centre
(b)
daughter cells which are dispersed to the
periphery.
Meristems may be functional for long periods of
time so that stem / daughter balance must be precise.
Basic body plan has been established by a
combination of WUS and CLAVATA3 (CLV3).
WUS is a transcription factor – which tells
cell adjacent to stem cells to express CLV3
Secreted CLV3 interacts in CLV1 / CLV2
receptors which limits WUS expression.
Control Circuit – Negative feedback Loop
![]() STEM CLV3
STEM CLV3
![]()
![]()
ORGANISER CENTRE WUS via
CLV1/CLV2
-
WUS knockout – meristems shuts down.
-
CLV3 knockouts – the meristem enlarges
Cell-to-cell communication is important, as if
the organiser centre cells are killed, adjacent cell will differentiate.
Root Meristem
Hypophysis will become the root quiescent centre…
![]()
…..which organises the meristem.
Organisation in established by cell-to-cell
communication mediated by auxin.
Evidence….embryo’s lacking auxin response
factor – MONOPTEROS fails to form hypophysis and no root tissue develops.
Is a similar feedback loop to WUS/ CLV3
involved in roots?
Not known but organisation is the same.
From meristems to organs
Leaves are typically produced directly from primary shoot meristems. …..daughter cells enter “zone of competence” where new organs form.
Leaf primordial derive from ~12 primordium
founder cells..located on the sub/epidermal layer at the periphery of the
meristem.
Leaf primordial formation is auxin mediated.
Cells between stem and periphery end up being
“stem”.
SHOOTMERISTEMLESS (STM) –
Prevents cells entering differentiation pathway
but repressing “ASYMMETRIC LEAVES1” (AS1).
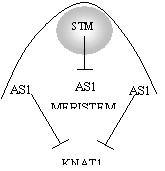
STM1 minus plants, AS1 is expressed in the
meristem which shuts it down
AS1 minus mutants KNAT expression persists in primorida resulting in leaves with a stem like appearance.
AS1/STM1/KNAT are all transcription factors.
Problem with
Culture: How do you mimic such a complicated process?
· Choosing the right explant.
o Inter-nodes
o leaf pieces
o zygotic embryos
o basal or apical meristems
o single cell suspension cultures
·
Altering the culture
conditions.
o changing hormone levels
o changing micro-nutrients
o changing pH
o
changing osmoticum
Problem with
tissue culture : Somaclonal variation
Tissue culture involves successive rounds of plant division.
With increasing time in culture, the regenerated plant
displays abnormalities e.g. albinism.
Genetic analysis revealed
considerable chromosomal re-arrangements.
Not due to "active
division" as not observed with meristematic tissue.
But callus tissue is also
stressed due to non-physiological levels of hormones are used.
But what is the cause of this somaclonal
variation?
Two main mechanisms are suspected..
1.
Plant
genomes are full of repetitive DNA.
This
repetitive DNA contains many examples of mobile genetic elements.
The
function of many
of these has
been lost over time........BUT some are activated during cell division and by
stress.
2. Could somaclonal variation
therefore be due to
Recombination between homologous regions.
To avoid
somaclonal variation,
1.
Must
avoid or reduce the period of tissue culture.
2.
Somatic embryogenesis
Surprisingly,
certain plant tissues can be induced to form "somatic embryos" which
are fundamentally very
similar to "zygotic embryos" which form within the seed.
A very attractive feature as
-
Makes regeneration easy - simply involving the "germination" of
the embryos.
- No
problem with somaclonal variation.
SOMATIC EMBRYOGENESIS
Somatic embryogenesis dependent only on auxins.
Very dependent on the right explants
- carrot single cell suspension cultures
- zygotic embryos
Appropriate auxin transport and distribution are needed for embryo development and pattern formation.
How is this controlled?
Activation tagging has identified “WUS” as a key element.