Plant Resistance
to Insect Grazing
1. Non-preference (Antixenosis) Insects choose not to feed or oviposit
2. Antibiosis Results in smaller adults or larval death
3. Tolerance
Antixenosis
1. Absence of an attractant
2. Presence of a repellant
3. Unfavorable balance between the two.
4. Tissue toughness
5. Nutrient deficiency
Canby is an aphid (Amphorophora agathanica) resistant raspberry. Phloem sap is deficient in sugar and nitrogenous compounds which are suitable for the aphid.
If only presented with the plant, mortality occurs in the first two intars.
Underfield conditions – aphids, probed and fed for no more than 24h before becoming “restless” and abandoning the plant.
6. Waxes
Major components of waxes are alkanes.
In rice, application of epicuticular wax from resistant variety IR64 to susceptible
IR22 detered probing by brown planthopper.
6. Structural deterrents
HAIR – PUBESCENCE
But some insects prefer “hairy” plants e.g. Hessian fly (Magetiola destructa)
Cotton bollworm (Helicoverpa zea) female hangs on to hairs when ovipositing
TRICHOMES act by
1) Simple impedance
2) Trap insects using – hooks or exudates from glandular Trichomes
Trichomes in Solanum, Lycoperscium, Nicotinia, oin contact with atmospheric oxygen produce a insoluble black substance.
Polyphenoloxidase and peroxidase activities – leading to oxidative cross-linking.
Plant Resistance to Insect Grazing : Secondary Metabolism
1o Metabolites Nucleotides, lipids, organic acids – produced by all organisms.
2o Metabolites Specific chemicals “tailored” to the need of specific organism.
2o Metabolites
1. Made during certain developmental phases of the plant.
2.Restricited to specific storage sites.
Intracytoplasmic -vacuole (eg. cyanogenic glucosides) and plastids (in chloroplasts ; phenylpropanes and flavanoids)
Extracytoplasmic cell walls, sub-cuticular spaces. Terpenes, generally restricted to resin ducts and glandular hairs.
FUNCTION OF 2O METABOLITES
Plant Growth Hormones auxins, cytokinins, ABA, gibberelic acid
Insect Attractors colour favanoids and carotenoids (see Insect Host selection)
Insect Repellents e.g terpenoids
TERPENOIDS
These compounds are not induced following wounding but following insects damage.
Terpenoid diversity is a major source of the distinctive “signature” – associated with each insect/plant interaction.
The
signature is produced either
(i)
insect encoded enzymes
e.g.
spider mites (Tetranychus urticae) produce (3S)-(E)-nerolidol synthase
(1) to release monoterpene 4,8-dimethyl – 1-3(E) 7 nanatriene (2).
This attracts the predatory mite Phytoseiulus
persimilis.
(i)
Insects producing
elicitory compounds.
e.g. Manduca sexta
induced the synthesis of mono- and sesquiterpenes as well as a
jasmonate burst.
Examination of M. sexta regurgitant identified fatty-acid – amino acid conjugates which
could elicit the “feeding signature” from Nicotinia attenuata.
Some monoterpenes are directly toxic to insects –
Very varied group derived form acetyl CoA or glycolytic intermediates. –
Acetate-mevalonate pathway.
Form by the fusion of 5 carbon “isoprene units” thus the
isoprenoid pathway → trepenoid pathway.
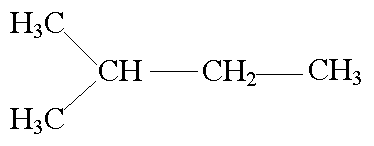
Derived from the condensation of three molecules of Acetyl CoA.
A series of complex polymers are based on this isoprene unit.
MONOTERPENOIDS. C10 terpenoids (2 x C5)
Volatile → giving plants their characteristic odor.
The “essential oils” – menthol, camphor and a-piene are all monoterpenes
LIMONENE and GERMINOL are constituents of flower scents – attracting wind pollinators.
PYRETHROIDS – found on leaves and flowers of Chrysanthemum species. These are neurotoxins which cause hyperexcitation, unco-oridinated movement and insect paralysis.
Synthetic analogues are now important commercial insectides
CITONELLOL is an oviposition-deterrent to leaf hoppers (Amrasca devastans)
SESQUITERPENOIDS C15 terpenoids (3 x C15) –
Can be either aliphatic or cylic.
1. DRIMANE SKELETAL TYPE - most potent insect feeding deterrents
e.g. POLGONIOL from Polygonum
WARBURGANOL from Warburgia.
Blocks the stimulatory effects of glucose, sucrose and inositol on chemosensory receptor located on insect mouthparts.
2. SESQUITERPENE LACTONES largest groups of sesquiterpenes e.g. alanolactone
Poisonous to lepidopterans, beetles and grasshoppers – functions by an unknown mechanism.
3. GOSSYPOL – a sesquiterpene dimer.
Toxicity arises from complexing with proteins in the insects gastrointestinal tract – inhibiting enzymic activity.
4. PHYTOJUVENILE HORMONE
Insect juvenile hormone has a farnesol backbone. Depending on substituting groups JH is classified into three groups.
Type III JH is closely related to the plant sesquitepene –JAVABIONE
The JH –mimicking effects of plant terpenes was first noted in when the cotton bug
(Pyrrhocoris apteru) persistently moulted into the nymph form when cultured with paper pulp from trees.
5. ANTIJUVENILE HORMONES
Precocene I and II from Ageratum houstoniatum cause precocious metamorphosis and sterilization on Hemiptera, Homoptera and Orthoptera.
Inhibits JH secretion from the corpus allatum in the insect brain.
DITERPENOIDS C20 (4 x C5) – “diterpenes” (includes gibberellins).
Non-volatile found in the resin of higher plants,
Insect resistance factors which increase insect mortality, reduce growth and inhibit feeding.
TRITERPENOIDS C30 (6 x
C5)
CUCURBITACINS extremely bitter tasting
SAPONINS, triterpenoid derived glycoside. Amphipathic – acting as a detergent. Binds to free sterols in the insect gut to reduce uptake into the hemolymph.
Reduction in steroids may interfere in insect moulting hormone-.
STEROIDS
Triterpenoid derived. Non-polar.
Phyto-ecdysome has been discoverted which severly inhibits growth, development and reproduction. Not lethal but affects insect fitness.
Nitrogenous protective compounds
Not in themselves toxic but are readily broken down to give off poisons. Two major examples -
CYANOGENIC GLYCOSIDES
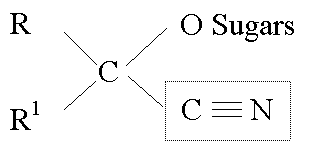
Broken down to give off cyanide (HCN) by a combination of glucosidases (in the vacuole) and hydroxynitrate lyase (cytoplasm).
These enzymes only mix when the cell is ruptured e.g following insect grazing.
GLUCOSINOLATES
In
Brassicas, isothiocyanates. are produced from glucosinolates and are
systemically emitted

The distinctive, pungent flavor and odor of mustard, radishes and horseradish is due to isothiocyanates.
Mustard Oil interferes with insect plant selection at two stages
(i) Pre-digestive – repelled by pungent odour
(ii) Post digestive – direct toxic effects.
GLUCOSINOLATE HYDROLYSIS
Glucosinolate is degraded by an
enzyme - myrosinase
Which depending on the reaction
conditions (pH primarily) will give off either isothiocyanates
![]()
![]()
![]()
![]()
![]() R
N C S
R
N C S
Or nitriles.
![]()
![]()
![]()
![]() R C
N
R C
N
Myrosinase is compartmentalized (where) until released following wounding.
Isothiocyanate deter grazing EXCEPT –
Cabbage white butterfly is attracted by glucosinolates and they are required for egg laying.
HOW DO INSECTS OVERCOME DEFENCE MECHANISMS?
DETOXIFICATION:
(I) Multifunctional oxidases present in the insect gut.
(II) During oviposition - wool wasps (Sirex sp. ) inject a toxin
a. inhibits polyphenoloxidases. and
b. spores of a symbiotic fungus into the leaf. Th elatter grown into conductive tissue to- blocks photosynthate translocation from the leaves a
BEHAVIOURAL CHANGES . Only feed for 1-2h, not allowing plant defences to build-up.
HOST TOXIN SEQUISTRATION
1. Western pine beetle (Denroctonus breviconis) is attracted to its host by the terpene –OLEOERSIN -. Females attract male by producing a pheromone – MYRCENE which is derived from oleoresin.
Therefore the population is proportional to the amount of food available.
When the population is optimal – females secrete VERBENONE a male repellent substance.
2. Larvae of Monarch butterfly (Danaus plexippus) accumulate CARDENOLIDES from milkweed. Larvae can regurgitate gut fluid with cardenolides – bitter tasting steroid – a potent regurgitant.