A Study of Sclerochronology by Laser Ablation
ICP-MS:
Do seashells hold the key to global warming?
Harry Toland, Dr Bill
Perkins, Dr Nick Pearce, IGES, University of Wales, Aberystwyth, Ceredigion,
Wales, SY23 3DB U.K
Fergus Keenan, VG Elemental,
Ion Path, Road Three, Winsford, Cheshire, CW7 3BX U.K
Dr Melanie J. Leng,
NERC Isotope Geosciences Laboratory, British Geological Survey, Keyworth,
Nottingham, NG12 5GG
Introduction
The
rhythms of life have been recognised and recorded for millennia. These
rhythms are mirrored in the world around us. This study hopes to shed light
on one of these phenomena, the recording of elemental signatures in the
repeated structures of marine organisms and interpreting these signatures
so that we may recognise the environmental parameters that existed when
they were formed.
Biogenic
carbonates have been the subject of countless studies for many years. Whether
to ascertain pollutant levels, elemental signatures and organism age or
to make use of repeated structures within the hard parts of the organisms
to unravel periodicity of elemental or isotopic concentrations.
The
marine bivalve Arctica islandica has been studied extensively and
the occurrence of annual growth increments, within Arctica, is widely
accepted. Arctica is found in temperate and boreal waters on both
sides of the Atlantic.They
are limited by a temperature regime of 0-19oC and a depth range
from below lowest low tide and ~
500 m. They can live for over 200 years. This periodicity is manifest in
the appearance of recognisable light and dark layers or growth bands within
the shell structure of the organisms aragonitic carapace. The light opaque
layers are generally laid down in periods of rapid growth from spring to
early summer while the dark translucent layers are laid down from late
summer to winter.
CaCO3
extracted from the seawater and incorporated into the shells of these organisms
is never pure and so leads to the inclusion of minor and trace elements.
In this study, levels of strontium, magnesium and barium were measured
in the growth layers of marine bivalves in order to investigate possible
links with ocean temperature and the process of primary production.
Allied
with the trace element signature within the aragonite, stable isotope analysis
can be equally rewarding. The technique relies on the natural occurrence
of the triple oxygen isotopes. When the climate is warmer the increased
evaporation effectively depletes the oceans of 16O, while simultaneously
increasing the levels of 18O. For open ocean salinity, approximately
0.2% fluctuation in stable isotope concentration occurs within the substrate
for every 1°C
fluctuation in temperature within the water. As the biogenic mineralisation
is in equilibrium with the surrounding waters changing temperatures within
the water will be mirrored within the shell. This study investigates the
variation trace metal content with stable isotope ratios in growth layers
of Arctica.
Materials
and Methods.
 Live
Arctica
islandica (Linnaeus), were collected on the 17th - 22nd
Feb. 1997 and 14th April 1998, from Borth sands, near the centre
of Cardigan Bay, Wales, (grid ref. SN 603 930). Cardigan Bay is a shallow
embayment in the south-eastern Irish Sea, which receives Atlantic ocean
water from the south. Arctica were collected immediately after a
storm, which dislodged the organism from their habitat further out at sea.
Living tissue was removed from the shells and the shells were washed and
scrubbed thoroughly in de-ionised water, to remove external contamination
and baked in an oven at 45oC for several hours, to remove any
trace of remaining organic material (fig 1&2).
Live
Arctica
islandica (Linnaeus), were collected on the 17th - 22nd
Feb. 1997 and 14th April 1998, from Borth sands, near the centre
of Cardigan Bay, Wales, (grid ref. SN 603 930). Cardigan Bay is a shallow
embayment in the south-eastern Irish Sea, which receives Atlantic ocean
water from the south. Arctica were collected immediately after a
storm, which dislodged the organism from their habitat further out at sea.
Living tissue was removed from the shells and the shells were washed and
scrubbed thoroughly in de-ionised water, to remove external contamination
and baked in an oven at 45oC for several hours, to remove any
trace of remaining organic material (fig 1&2).
The
selected valves were strengthened by filling with resin and allowing them
to harden. The valves were then sectioned into 10mm slices from the umbo
to the ventral edge, crossing all growth increments. Finally the sections
were polished and cleaned in an ultrasonic bath. Acetate peels were prepared
from these sections and the numbers of annual growth increments were counted
for each section. Shell sections with the most discernible annual increments
were selected from each of the samples and labelled LB2, LB5, LB6, LB7
and LB8.
Analytical Techniques
Stable
isotope analysis

Samples were taken at
intervals of 0.5mm along the cross section between the umbo and the ventral
edge (Figure 3), using a 0.5mm diameter drill. Approximately 0.2mg of calcium
carbonate was removed from each hole drilled. The powders were then transferred
to the NIGL stable isotope laboratory in Keyworth, where stable isotope
analysis was performed. 100
mg
portions were analysed in a VG Isocarb+ Optima mass spectrometer system
together with a similarly sized sample of a laboratory calcite standard.
Results are reported in the usual d18O
and d13C
notation in per mill (‰) versus VPDB, based on calibration of the laboratory
standard against NBS-19. Analytical precision (1 SD), based on the laboratory
standard is typically <0.07‰for both d18O
and d13C.
Laser
Ablation ICP-MS
Due
to the sample size required for solution ICPMS and the nature of the annual
growth increments within Arctica (can vary in size from mm to 10’s
of microns), analysis by solution ICPMS can result in a homogeneity of
elemental signatures from several years. Laser Ablation ICP-MS has a spatial
resolution of <10µm and is therefore the ideal tool to differentiate
between the yearly growth increments which can be of the order of 10's
µm in Arctica.
 Analysis
was carried out at the stableisotope
drill sitesusing the VG
MicroProbe II
and PlasmaQuad
3 laser
ablation ICP-MS system.
Analysis
was carried out at the stableisotope
drill sitesusing the VG
MicroProbe II
and PlasmaQuad
3 laser
ablation ICP-MS system.
The
instrument was tuned and optimised using NIST glass standards 610 &
612and BCR-CRM 393(pressed powder).
Each
stable isotope micro-drill hole was sub-sampled by laser ablation, either
by single spot ablation or using a continuous raster pattern. Gas blank
measurements were taken before and after the analysis and standards were
analysed at the beginning and end of the analysis.
Scanning
Electron Microscopy
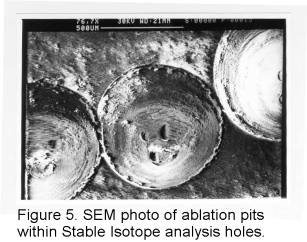
The shells were removed from the analysis chamber and washed in a static
bath and air dried. A number of shell sections were then prepared for the
scanning electron microscope (SEM). The sections were mounted on an aluminium
stub with glue and allowed to dry. The sides of the sections were painted
with silver paint, designed to earth the section to the stub and thus negate
any charging of the section within the SEM chamber. SEM images were then
taken with a 35mm SLR camera mounted onto the instrument. These images
(Figure 4 and Figure 5), show the difference in resolution between stable
isotope analysis and LA-ICP-MS.
Results.
The
stable isotope analysis was taken from the periostracum edge of the shell
section within the annual increments (Figure 3). The results are shown
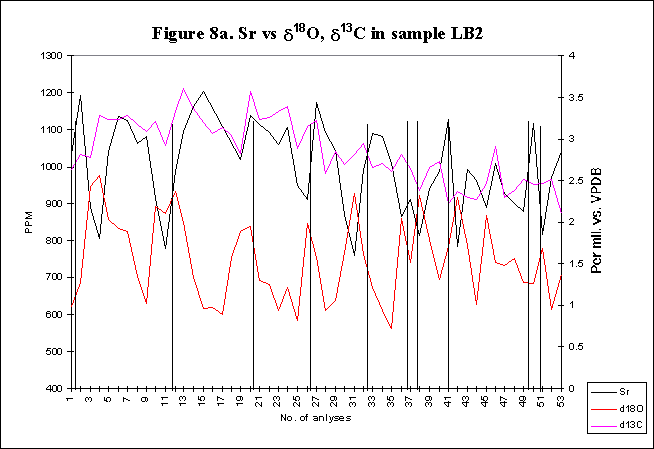
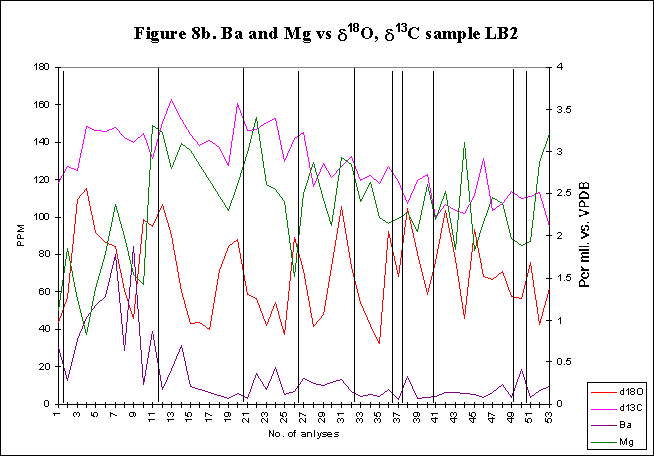
graphically in figures 8 - 12.
Each
of the shells was sectioned at different positions and results will therefore
reflect different suites of successive years of growth within Cardigan
Bay. The x-axes in the graphs below denote the number of analysis sites.
Analyses increase sequentially from the younger portion of the shell to
the older. The primary y-axis denotes the elemental concentration in PPM
while the secondary y-axis denotes the stable isotope signals, d18O
and d13C.
The vertical lines within graph LB2 indicate the position of the start
of successive growth increments within the shell section. Distances between
the vertical lines equate to the size of any one growth increment (the
equivalent of one year). Empty cells have been interpolated.
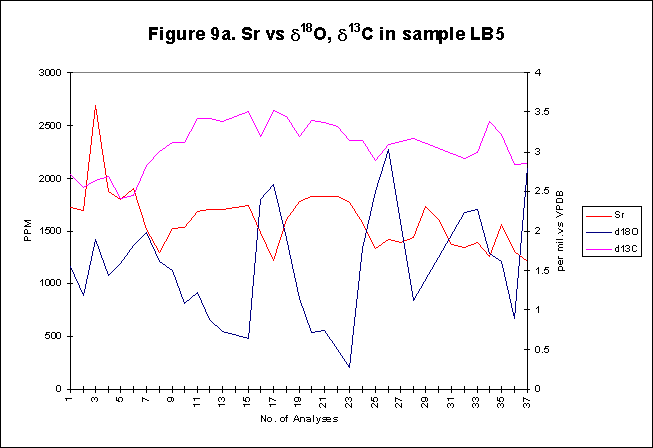
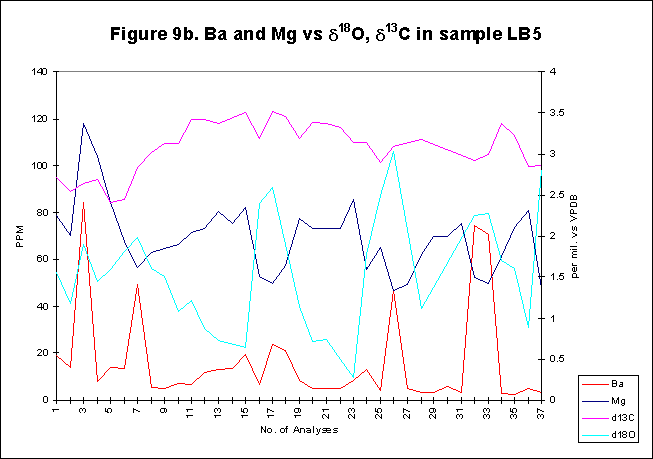
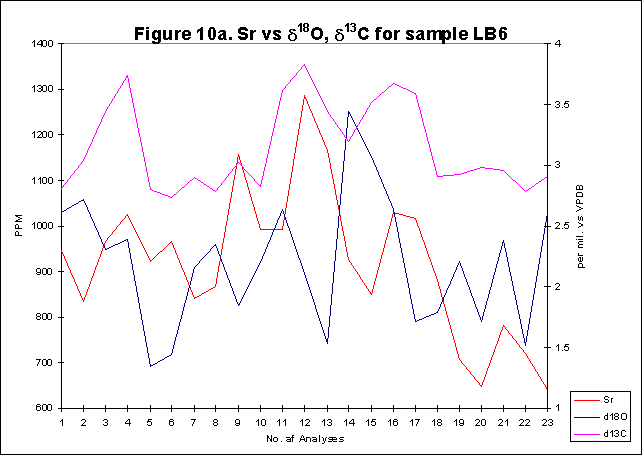
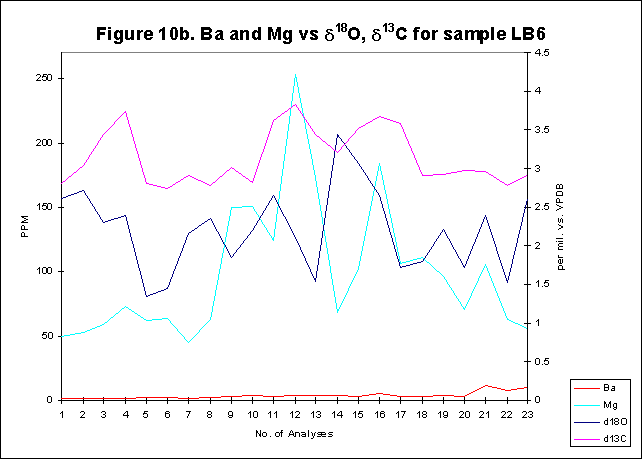
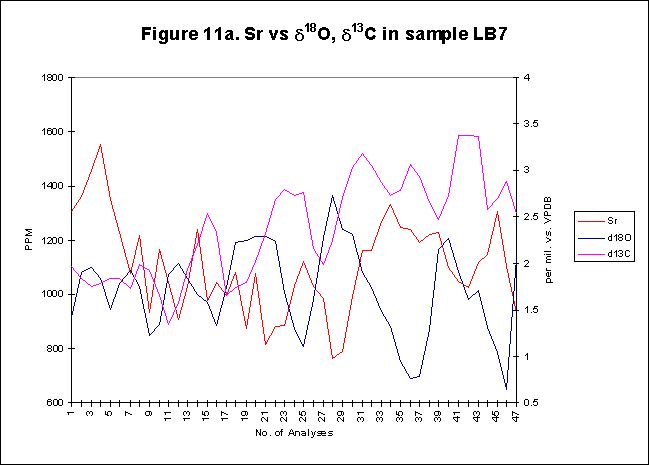
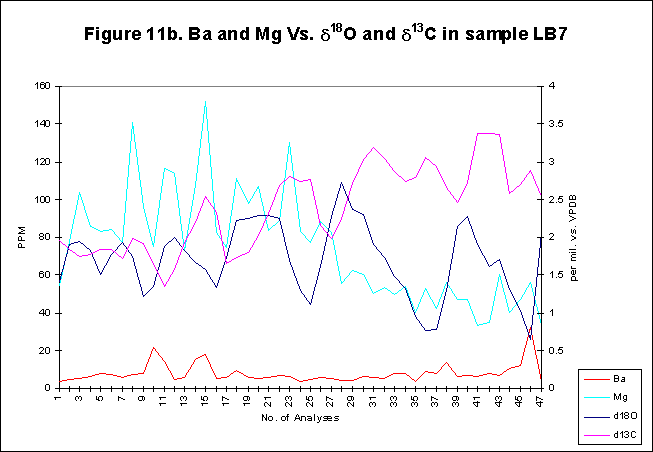
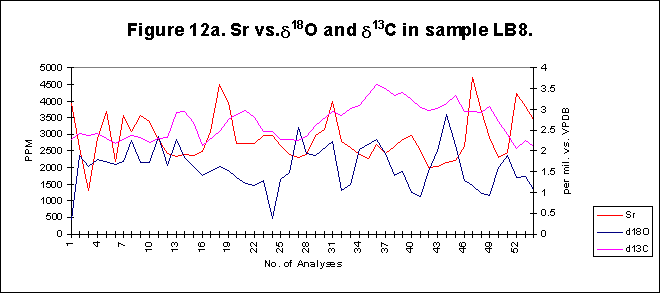
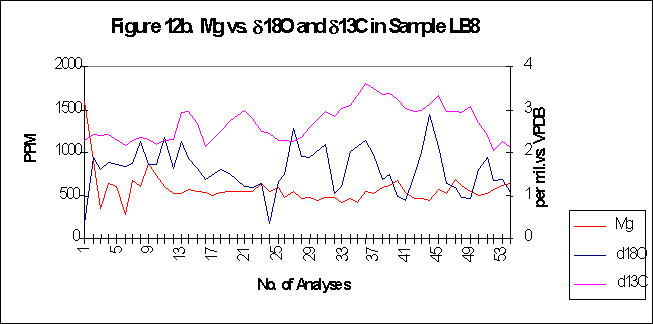

The levels of
d18O
and d13C
within the shells are indicative of open estuarine conditions with a throughput
of fresh Atlantic waters. The range of values is from ~0.5-3.50/00,
for d18O
and from ~1.5-3.50/00
ford13C.
These data sets reflect variations in temporal, spatial, ontogenetic and
gamontogenetic conditions. Both exhibit cycles which have a sharply rising
leading edge, coupled with a gradual fall towards the next lowest value.
The
elemental signals exhibit a strong cyclic variation. Sharply rising leading
edges and gradually falling trailing edges are also evident within the
elemental concentrations. Sr concentrations range in value from ~600-4500ppm,
Mg varies from ~50-1500ppm,
Ba has a range of ~5-80ppm.
Discussion
The
ability to see repeated patterns within biogenic structures enables the
researcher to put forward definite arguments based on clearly perceived
data. Arctica Islandica is the perfect tool for recording environmental
conditions which existed at the time of its biogenesis because:
1.
it is reported to live for up to 220 years
2.
it exhibits yearly growth structures
3.
it has a wide boreo-atlantic range
As
Arctica is primarily a filter feeder, filtering algae from the water
column., it follows that a relatively large volume of seawater passes through
the organism. The constituents abstracted for shell formation (Ca2+,
Mg2+, Sr2+, CO32-), are either
ingested as food or formed as metabolic products of respiration. The concentrations
of trace elements and the levels of stable isotopes incorporated in the
shell vary according to the conditions that exist at the time of crystallisation.
These conditions will reflect the ambient sea conditions at that time.
Figures
8-12 show d18O
values over a number of years within sections of individual Arctica
shells. As discussed above d18O
levels can be directly related to temperature. It has been well documented
that d18O
values have an inverse relationship with temperature in biogenic carbonate.
The d18O
variation of ~3‰
is within the expected range for shell carbonate. Ambient seawater temperature
is estimated from derived d18O
values within aragonite using the formula below:
·Formula
1.[19.0- (3.52(OA-OW))+0.03(OA-OW)2]
WhereOA
is the concentration of d18O
in the sample
OW
is the concentration of d18O
of seawater,
(Assumed)
to be 0/00
Transposing
the highest and the lowest values for d18O
for each shell into the formula we see that:
These
are estimates of highest summer temperature and lowest winter temperature
for each shell between the first and last year analysed. These temperatures
compare well with the measured range of sea surface temperatures for the
area of study (Table 1).
Table
1. Mean Sea temperatures for
Moelfre (°C). Pos. 53 21N4 14W
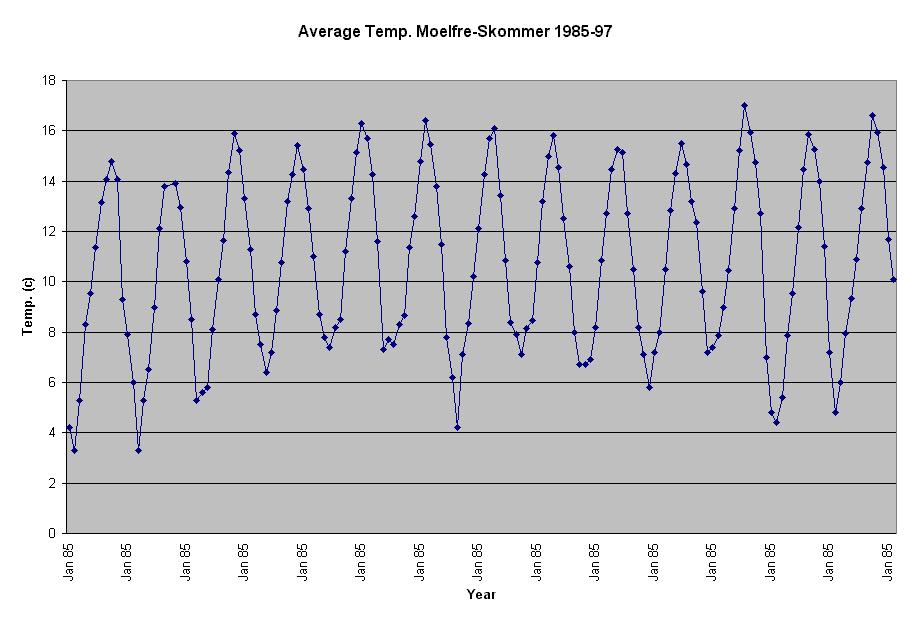
Table 1 Average temp 1985-1997 Cardigan Bay.
Vertical
lines in Figure 8 denote growth increments. Each vertical line coincides
with dark banding within the shell, which corresponds to winter growth.
Clearly these winter growth lines are proximal to the maximumd18O
values for each yearly cycle. This reinforces the principle that growth
increments within Arctica are indeed annual. Growth increments can
and do fluctuate in size over the life of the organism. This fluctuation
in demonstrates that growth is dependent on variables such as environmental
conditions, spawning, disturbance, pollution and ontogenetic differences.
While it is typical for greater growth potential to be realised when the
organism is in its younger stages, it is not an absolute rule and relatively
larger growth increment do occur sporadically in later life, dependent
on conditions. However the commencement and cessation of each d18O
cycle is temperature dependent. Each growth increment has one major cycle
within. Superimposed upon this major cycle are minor variations. These
variations may be due to the influence of other factors, such as salinity,
brought about by freshwater surges or by the cessation of food intake with
the onset of spawning. This in turn may cause small fluctuations in the
stable isotope concentration within the biogenic aragonite laid down by
the organism. The d18O
signals have steep angles of rise and shallow angles of fall. This may
be due to mismatching rates of isotopic incorporation and loss within the
aragonite, brought about by seasonally adjusting temperatures.
A
similar theme of annually adjusted d13C
values appears to be encountered within the shells. The relationship between d13C
and primary production is characteristically inverse, with high productivity
at the surface, mirrored by high dissolution rates at depths. The introduction
of isotopically light C, increases the ratio of 12C to 13C,
thus decreasing d13C-values.
Most of the surface waters in the central ocean basin have d13C-values
of ~2.20/00.
Bivalves may or may not secrete d13C
within their shells in equilibrium with the ambient seawater. It is thought
that the d13C-value
of their shell (calcite) may reflect that of the surrounding water, though
metabolic rate and/or salinity within the surrounding medium can exert
an influence on measured concentrations. The higher d13C-value
typically indicates periods of reduced primary production, while lower d13C-values
indicate a period of increased primary production.
A
definite cyclicity within years can be observed. Growth increments (1 year)
are characterised by early to mid year increases in d13C-values,
perhaps reflecting annual blooms within Cardigan Bay, or periods of increased
metabolic activity. The variation of d13C-values
within years, while pronounced is generally relatively small. The overall
trend of d13C-values
is upward, with the exception of LB2, which decreases as the number of
years crossed increases. This discrepancy could be due to availability
of food (more food towards the latter years), or to a change in the metabolic
rate/efficiency of the organism.
The
high spatial resolution afforded by LA-ICP-MS coupled with detection limits
in the ppb range allows accurate analysis of individual growthincrements
which can be as small as 10's µm in Arctica. Strontium, Magnesium
and Barium levels were analysed by LA-ICP-MS, within the same sections
of Arctica previously analysed for stable isotopes.
Variations
of strontium within biogenic carbonates have been linked to temperature,
salinity, kinetic controls, metabolic controls, ontogenetic effects, gamontogenetic
effects and calcification rate. The present study suggests that strontium,
within Arctica, varies in response to seasonal changes. Comparisons
drawn between Sr and stable isotope analysis point towards seasonally adjusted
temperature as the dominant control on strontium distribution within growth
increments in Arctica islandica.
Each
of the analysis points on the graph represents the mean of three laser
ablations from within the stable isotope drill holes (Figure 3). The Sr
profiles produced by the analysis, varies cyclically around the mean by ±~500-1000
ppm, for most shellfish, in the early years of life. In most cases the
magnitude of this variation falls as the organism gets older, indicating
either that growth exerts an influence on Sr incorporation, or mantle metabolism
is biasing Sr incorporation.
In
general the signal within each growth increment is characterised by a mid
year peak, with beginning and end of year lows. Ostensibly this mirrors
seasonally adjusted temperatures with relatively cold winter water warming
from the spring through summer then cooling again as autumn comes and winter
returns.
d18O-values
versus Sr concentration, all against time, are recorded for each shell.
Each stable isotope analysis has been micro-drilled along the length of
the section, adjacent to each other. Each point in the Sr concentration
signal represents the average of 3 ablations
within the hole created
by the micro-drilling (Fig F). The strontium levels recorded are in keeping
with other studies, on similar matrix. Averages of 1200-1500 ppm strontium,
within Arctica, from relatively warm shallow waters such as Cardigan Bay
are to be expected.
The
most striking feature of all the graphs is the strong inverse relationship,
which exists between the two signals. High Sr concentrations in the summer
months are opposed by low d18O-values
over the same period. Minor variations within the Sr concentrations are
balanced by opposing but similar variations of d18O-values.
The inverse nature of the relationship between the two signals provides
a useful tool, one, which can be used to determine seasonal footprints
within shells. Highs in Sr levels equating to summer, warming temperatures
and lows relating to winter cooling temperatures. There is a slight lead
in phase of Sr signal compared to the stable isotope signal, this has been
noted
by previous investigators, who were unwilling, due to spatial resolution
constraints, to interpret. With the improved resolution of this study,
greater confidence in the authenticity of a Sr phase lead can be assumed.
It may be that mollusc biological controls are responding to seasonal changes,
with instantaneous Sr variation, while d18O-levels,
responding to temperature variations are less immediate. It appears feasible
to employ Sr concentrations as direct proxies for d18O-values,
which in turn are representative of ambient seawater temperatures.
While
the association of Mg concentrations with temperature has been well established
in bivalve, direct cause and effect have not. Mg has a tendency to vary
with temperature with “probable exceptions”. It has been recognised that
salinity, growth rate, mineralogy and temperature can effect Mg concentrations.
The
present study area (Cardigan Bay), has a fairly constant salinity regime
and therefore should not adversely influence the constituents of any biogenic
carbonates precipitated within it. There is no consensus within the field
of biogeochemistry as to the causes of Mg variations within biogenic minerals,
however this does not preclude inferences being drawn and relationships
recognised between spatially and temporally similar data.
Magnesium
concentration within Arctica, analysed by LA-ICP-MS varies around
relatively constant means of ~100
ppm, for each sample, with the exception of LB8, which has a much higher
mean of ~500
ppm. The fluctuations around the mean Mg value are also relatively constant,
with a value of~100
ppm. This may be due to changes in temperature. The changes in signal amplitude
appear to be abrupt yet cyclical, corresponding with the phenomenon of
gradual temperature change as the heat budge increases/decreases. Seasonality
may be the key to understanding Mg concentrations within biogenic minerals.
Individual inputs to the system may change from season to season, increasing/decreasing
the concentration of Mg within the system, but the overall variation around
the mean remains constant.
Magnesium
and d18O
are well established as reliable proxies for temperature within biogenic
carbonates. The graph of the two signals exhibits a linked relationship.
Within the sample, Mg maxima and minima tend to lag those of d18O.
Mg within calcareous organisms is strongly controlled by ambient water
temperature. As Arctica is a benthic dweller it follows that the
ambient sea floor water temperature within which it lives, will always
be at variance with sea surface temperature (by ~1-2°C).
Mg within Arctica will reflect these sea floor temperatures, while d18O-values
reflect sea surface temperatures, and therefore seem to lead to Mg maxima
and minima. Similar analyses have found that d18O-values
exhibited anomalous tendencies when salinity levels within the water-mass
fluctuated, while Mg values were unaffected for the same salinity. If this
is the case, then d18O-values
for Arctica, within the present study, may be overestimated in some
analyses, while Mg concentrations may reflect a more accurate dip in temperatures
over the same period.
Barium
has been shown to be an effective tracer for regions of the oceans with
high primary productivity. In previous study’s, correlation’s have been
drawn between Ba and primary production in molluscs. It has been postulated
that increased in Ba/Ca ratios in Mercenaria mercenaria represent
sudden influxes of barite to the sea floor from phytoplankton blooms above.
Applying this hypothesis to the Ba data from the present study it seems
likely that a relatively large amount of Ba was incorporated into the shell
of Arctica within some years, while others had very little, which
may indicate a relatively large phytoplankton bloom in that year. The signal,
with its sharp rises and falls may indeed be tracking phytoplankton blooms,
with ephemeral and short lived ‘superblooms’ forming irregularly, overriding
usual background levels of Ba production. The majority of Ba activity within
the shells seems to occur after the start and before the end of most years,
corresponding to the time of year when optimum bloom conditions exist.
Barium
and d13C-values
are both reported as being proxies for primary production within the water
column. Ideally an inverse relationship between the two signals should
be conspicuous, however, this is not always the case. Both signals exhibit
in phase maximum values at times, both signals feature a fall off in concentration
at other times. In general Ba and d13C
concentration follows the trend predicted with highs in Ba mirrored by
lows in d13C,
however occasionally this relationship breaks down completely. The reasons
for this deviation remain complex but may be explained by the anaerobic
habit of Arctica, i.e. the phenomenon of anaerobic respiration,
which Arctica has been recorded to practice. If and when the relationship
betweend13C
and Ba breaks down, it may be due to anoxic conditions within the environment
of deposition. Increased d13C-values
within anoxic sediments have been reported, this may positively influence
the incorporation of d13C
into the carapace of Arctica, increasing each summer as anoxic conditions
returned to the environment of aragonite deposition.
5.
Conclusion
Arctica
islandica,
the Ocean Quahog, may be the oldest living invertebrate known. Because
of this and the fact that Arctica, like many other bivalve molluscs,
precipitates it shell, with an annual periodicity, in equilibrium with
the surrounding seawater, it is an excellent chronicler of ambient environmental
conditions. The advent of high spatial resolution LA-ICP-MS now makes it
possible to extract elemental information from the smallest of growth increments.
When elemental analysis by LA-ICP-MS is allied with the proven technique
of stable isotope palaeo-themometry, deeper interpretation of relationships
and trends within and between results can be achieved. Loose assumptions
can be tightened up and data can be relied on. The following observations
were made in this study:
·Sr
exhibited a strong inverse relationship with d18O-values
and as such may prove to be a reliable indicator of ambient sea temperature.
·Mg
concentrations, while not exhibiting as strong a relationship with d18O-values
as Sr, nonetheless did respond to changes in d18O-values
and as such confirmed its effectiveness in reconstructing environmental
conditions.
·The
results obtained for Ba concentration within the shells, suggest that this
element may be applied as an effective proxy for primary production within
molluscan carbonate.
LA-ICP-MS
and the apparent suitability of elemental signatures as proxies for environmental
conditions warrant further study and interpretation.
While it is a complimentary technique to stable isotope measurement, LA-ICP-MS
has several key advantages:
·Spatial
resolution is improved by a factor >20 while precision is kept high.
·LA-ICP-MS
is less susceptible to salinity variations
·Sample
preparation time is reduced significantly, as is analysis time
·Costs
are considerably less for the same number of analyses
Future
work will involve the use of LA-ICP-MS to analyse Arctica islandica
and Mercenaria mercenaria from quaternary sources. Data recorded
will be compared with analyses from contemporary shellfish and interpolated
with data from mollusc fossils in an attempt to reconstruct paeleo-environmental
conditions.
 Live
Arctica
islandica (Linnaeus), were collected on the 17th - 22nd
Feb. 1997 and 14th April 1998, from Borth sands, near the centre
of Cardigan Bay, Wales, (grid ref. SN 603 930). Cardigan Bay is a shallow
embayment in the south-eastern Irish Sea, which receives Atlantic ocean
water from the south. Arctica were collected immediately after a
storm, which dislodged the organism from their habitat further out at sea.
Living tissue was removed from the shells and the shells were washed and
scrubbed thoroughly in de-ionised water, to remove external contamination
and baked in an oven at 45oC for several hours, to remove any
trace of remaining organic material (fig 1&2).
Live
Arctica
islandica (Linnaeus), were collected on the 17th - 22nd
Feb. 1997 and 14th April 1998, from Borth sands, near the centre
of Cardigan Bay, Wales, (grid ref. SN 603 930). Cardigan Bay is a shallow
embayment in the south-eastern Irish Sea, which receives Atlantic ocean
water from the south. Arctica were collected immediately after a
storm, which dislodged the organism from their habitat further out at sea.
Living tissue was removed from the shells and the shells were washed and
scrubbed thoroughly in de-ionised water, to remove external contamination
and baked in an oven at 45oC for several hours, to remove any
trace of remaining organic material (fig 1&2). 

 Analysis
was carried out at the stableisotope
drill sitesusing the VG
MicroProbe II
and PlasmaQuad
3 laser
ablation ICP-MS system.
Analysis
was carried out at the stableisotope
drill sitesusing the VG
MicroProbe II
and PlasmaQuad
3 laser
ablation ICP-MS system.












