The Inflammatory
Response:
The
simple reddening of the tissues, which is seen when e.g. the skin in wounded is
in fact the outcome of a very complex regulatory process.
The
inflammatory response acts to
·
seal the site of injury
·
prevent
opportunistic infections
·
neutralize
established infection
Overview
Tissue
based “startle” reaction to trauma
Initiation
of activating proteolytic cascades -
“Go signals”
Molecular
cues for the beckoning, instruction and dispatch of leucocytes
Killing
of microbes and host cells they infect
Liquefaction
of surrounding tissue to prevent microbial metastasis
Healing
of tissues damaged by host response
The
inflammatory response may cause more problems than any infection.
Disorders in which an important pathogenic role is assigned to inflammation
Alzherimers
disease Multiple
sclerosis
Asthma
Psoriasis
Atherosclerosis
Rheumatoid
arthritis
Crohn
Diseases Type
I diabetes
Xenograft
rejection
Diseases where inflammation may contribute to the overall pathology
Bacterial
dysentery Influenza
virus pneuomonia
Cystic
fibrosis Leprosy
(tuberculoid form)
Heliobacter
pylori gastris
Tuberculosis
Hepatitis
C
BUT,
inflammation is usually life preserving
As
shown by patients which have a genetic deficenicy in a component to the
inflammatory process.
e.g.
inability to produce complement components predisposes to meningococcal
infection.
An important concept to grasp is that the inflammatory response occurs in two phases
·
a
proinflammatory stage and an
·
anti-inflammatory
stage.
..and
the same signal can have either effect…
What
governs the role of a signal is its context i.e. interaction with other
signals which are
derived from
]
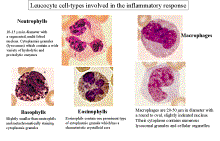
Cellular (Leukocytes) Components of
Inflammation.
Polymorphonuclear
cells
 Click on image for larger version
Click on image for larger version
Neutrophils:
·
Major
leukocytes present in bone marrow and blood.
·
Differentiate
under the influence of Granulocyte and Granulocyte-macrophage colony
stimulating factors.
May have medical role in e.g. chemo and
radiotherapy.
·
Terminally
differentiated
·
Rapidly
recruited to site of inflammation / infection
·
In
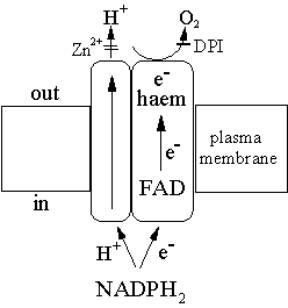
response to phagocytic stimulus undergo – an “oxidative burst”
Major antimicrobial and cytotoxic mechanism.
Produced by an NADPH oxidase. b558 cytochrome
Uses NADPH as a reductant provide electrons across the membrane.
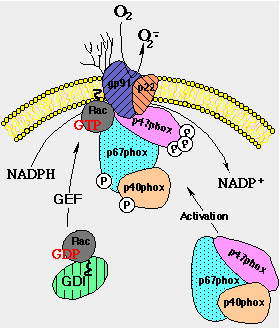
·
Recruited
cells undergo apoptosis.
·
Neutrophils-
the most motile of leukocytes- will accumulate first, though they have the
shortest “half-life” (6-72h).
After 12-48h macrophages
will predominate.
Eosinophil and
basophils
·
more
weakly phagocytotic than neutrophils.
Their roles are less well
defined.
Sentinel Cells – Mast Cells and Macrophages
Mast
cells are scattered in the skin and muscosa.
Originate
from a haematopoietic lineage
Circulate
in the blood and lymphatic system homing into target tissues.
Triggered
by
Allergens
complexed to IgE
C3a
and C5a anaphylabtoxins of complement system.
Perivascular
mast cells release
histamine,
eicosanoids,
(cause
vasodilation - responsible for heat and redness- and extravasation of fluid;
the cause of swelling).
pre-formed
“Tumour Necrosis Factor”. IL-1. Activates neutrophils initiating small amounts
of elastase.
Cleaves
the anti-adhesive coat of CD43 (leukosialin) allow integrins to engage in
endothelial extracellular matrix proteins,
Trytases
cleave protease-activated receptors
Neotermini
engage G-protein-coupled receptors the net effect of which is make endothelium
sticky for leukocytes and leaky to fluid.
Mutants
show that the following signals for switching from killing to healing.
Secretory
leukocyte protease inhibitor (SLP1) -
suppresses the release of elastase and ROI by TNF-stimulated neutrophils
TNF
Mononuclear phagocytes
(macrophages).
·
Made
in bone marrow before entering circulatory system. (A reserve is “retained”).
·
Once
reaching target tissue they have a life span of several months- macrophages.
Macrophages produce
·
Proteases,
hydrolyases, Oxygen free radicals
·
Complement
· Prostaglandins
and leukotrienes
·
Interleukin
1 (IL-1) and Tumour Necrosis Factor-a (TNF a)
Cellular
Events in Inflammation
The
major cellular event during inflammation is the movement of cells to the site
of injury.
Leukocytes
then become sticky, at first rolling over the inflamed endothelium following
adhering tightly before dispedesis (transmigration).
The
processes underlying each stage are increasingly well understood.
Chemotaxis
Local
injury results in the production of chemokines especially IL-8.
Side point: What are chemokines?
Chemokines
have
·
Leukocyte
chemoattractant
·
Cytokine-like
activity.
First
discovered when IL-8 was a monocyte derived neutrophil-specific chemotactic
factor.
No
previous chemoattractant was selective for specific leukocyte subtypes.
Key
directors of
(i)
specific leukocyte trafficking under emergency conditions such as inflammation.
(ii)
cell-type specific interactions with growth factors e.g.
·
Angiogenesis
– blood vessel development
·
Hematopoiesis
– blood cell development in bone marrow
(iii)
Anti-viral agents .
Certain cytokines can suppress HIV-1
interaction with
specific chemokine
receptors of leukocytes that are important for viral
entry.
Showed that some microbes act my molecular
mimicry.
What makes a chemokine?
Defined
primarily by structural criteria –
·
Single
polypeptide chain 70-100aa
·
25-95%
amino acid sequence homology
·
Conserved
cysteines – used to distinguish four main subfamilies
C, CC, CXC and CX3C
·
Lymphotactin
– only member of the C family
·
Fractalkine - only member of the CX3C family
and which has a transmembrane domain allowing it to be tethered to the cell
surface.
Chemokine
Receptors
·
15
known chemokine receptor subtypes
·
bind
multiple chemokines
·
in
a sub-class specific manner
·
Seven
transmembrane G-protein coupled receptors
Other major attractants..
Complement
components (mainly C5a)
Kinins
Collagen
and fibrin breakdown products
Bacterial
(e.g. from pathogens) products, particularly lipopolysaccharide (LPS;
endotoxin).
The
attractants activates the cells locomotor apparatus and amoeboid movement (by
imitation of cytoskeletal movement).
Margination
Macrophages
produce IL-1, TNF cytokines which induce the expression of adhesion molecules-
SELECTINS.
Selectins
are carbohydrate-specific molecules involved in weak-reversible binding to
sugar moieties on the cell surface.
Selectins
are responsible for the rolling and margination of neutrophils.
Click
here
for a movie showing neutrophil rolling. 
 Click on image for larger version.
Click on image for larger version.
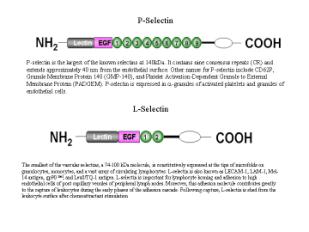
P-
selectin is a likely initiator of rolling.
In
P-selectin deficient mice, no rolling is seen.
Stored
in Weibel-Palade bodies of endothelial cells and is released within minutes of
treatment with such proinflammatory agents as histamine and thrombin.
L-selectins
– expressed in leukocytes and is involved in interactions with endothelial
cells. Responsible for continued rolling.
E-selectins
– are expressed in the endothelial cells and interact with neutrophil
carbohydrate.
Firm
binding is brought about by IL-8 induced expression of b2 integrins. These also
direct the arriving neutrophils to the site of injury.
Leukocytes
b2
integrin – heterodimeric transmembrane glycoprotein with a common b chain (CD18) and three a chains (CD11a,b,c).
b2 integrins also bind to the C3 component of complement.
Neutrophils
express elastase cleaves the anti-adhesive coat of CD43 (leukosialin) allow
integrins to engage in endothelial extracellular matrix proteins
Leucocyte
activation results in their “lining up” along the endothelial surface.
Emigration
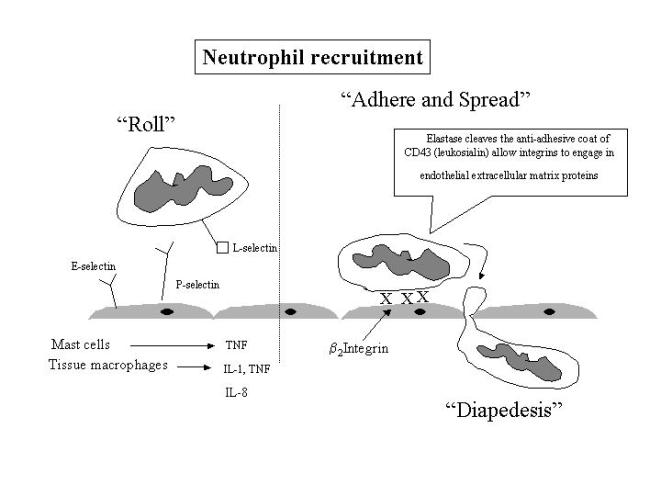
The
process through which leukocytes enter the perivascular tissue by moving
BETWEEN the endothelial cells.
Occurs
30-40 min after inflammatory stimulation.
Once
in contact with the endothelial cells they extend pseudopodia and migrate
through tissue.
The
cells migrate through intercellular junctions in the endothelium using a plasma
membrane glycoprotein (CD31) –
CD31
is a member of immunoglobulin superfamily which is expressed in neutrophils,
monocytes as well as the endothelium.
Hence
can get homophillic binding.
Results
in a dispedesis without leakage.
Plasma
leakage is a sign of acute inflammation.
Within
the tissue IL-8, C5a complement and leukotrienes are potent chemoattractants.
Ligate
to specific receptors on the neutrophil surface initiating cytoskeletal
re-organisation, respiratory burst as well as chemotaxis.
Initiating the
Inflammatory Responses -
Click on either image for a
larger version.
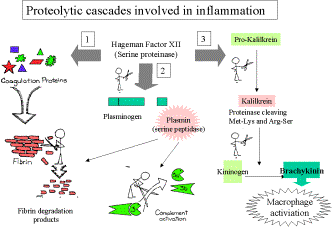
1. Plasma-derived mediators
Proteins,
which circulate in the plasma, usually in an “inactivated” form.
Hageman Factor (XII of the coagulation cascade)
Induced
by b-globulin
which is activated by contact with negatively charged surfaces –
·
Collagen
and basal membranes (i.e. wounded tissue).
·
Antigen:
Antibody complexes (e.g. infections).
Activated
factor XIIa
initiated three major processes…..
·
Coagulation
of blood proteins – fibrin formation.
·
Plasmin
activation
Plasminogen
cleavage releases plasmin; a broad specificity protease which degrades fibrin
(breakdown products increase vascular permeability) and activates complement
system.
Activates
more factor XII – positive feedback loop.
Generation
of kinins.
Bradykinin
is the major product of these reactions.
Increases
vascular permeability, smooth muscle contraction and “causes pain”.
ACTIVATES MACROPHAGES
The developing
Inflammatory Response : The Acute Phase
Characteristics
(i) Fever (hormonal interaction
with the hypothalamus in the brain)
(ii) Increased number of
circulatory neutrophils
(iii) Synthesis of acute phase
proteins – synthesized predominantly in the liver.
Gene
expression during the active phase has pro or anti-inflammatory
roles.
Control of blood clotting and repair Function
alpha-2
antiplasmin modulation of
coagulation cascade
Factor
VIII clotting formation of fibrin
matrix for repair
Fibrinogen
clotting formation of fibrin
matrix for repair
Fibronectin fibrin clot formation
Haptoglobin haemoglobin
scavenger
Heme
oxygenase heme
degradation
Cytokine-like Function
C-reactive
protein Activates complement
interaction with T-cells and B-cells
Kallikreins vascular permeability and
dilatation
Complement
and associated functions Function
C1
inhibitor negative
control of complement cascade
C2,
C4, C5 and C9 complement component
Plasminogen proteolytic activation of complement,
clotting, fibrinolysis
Proteinase
inhibitors Function
Act
to prevent the migration of leukocyte cells preventing the establishment of a
systemic
inflammatory response.
alpha-1
antichymotrypsinogen
alpha-1
antitrypsin
Plasminogen
activator inhibitor-1
Many
of the above pro- and anti-inflammatory proteins are regulated by cytokines
which are produced by activated macrophages.