The following must be addressed in the protocol:-
1: Important -> large number of regeneratable cells. This is usually
maximised by including a tissue culture step.
2: Optimising plant and Agrobacterium genotype-
It is not essential to transform "elite" lines but close relative that may be
crossed in. e.g. diploid wild strawberry -> Fragaria vesca ->cross with octoploid
edible strawberry.
3: Adding vir gene inducers such as acetosyringone
4: pH, temperature, light conditions
5: Length of co-cultivation
6: Hormones
7: Choice of explant type
8: Is wounding necessary? Ultra-sound may improve transformation
Transforming tobacco leaf disks (see slide show)
Transformation in planta: Arabidopsis using floral dip.
First tried
because egg cell receives entire genome from sperm cell – Is it therefore
also receptive to
T-DNA
insertion?
Possible targets: gametophyte or recently fertilised embryos.
Chop back first flowering: Dunk when second flowering "bolt" is 2->10 cm high.
Needs no hormones or culture steps; no problem with somaclonal variation
Needs a "surfactant" e.g. Silvet 77:
Enhances entry of bacteria into inaccessible plant tissues-
New techniques
are being developed where Arabidopsis plants are simply sprayed
with
Agrobacterium.
Few plant cells
are terminally differentiated and are totipotent:
Two
forms of plant tissue culture:
ORGANOGENESIS
AND SOMATIC EMBRYOGENESIS (more on the latter later).
Organogenesis
Relies on the ability ot most cells to "dedifferenetiate" and revert to a being "stem-cell".
i.e. actively dividing with no clear viariation in cell type.
In plants, this is refered to as formation of MERISTEMS and in culture is typhified by the
formation of callus tissue.
Various explants or suspension cell cultures can be used to induce meristemic tissue when cultures
with hormones (see handout).
By changing the in hormones in the culture medium it is possible to induce callus cultures to
re-differentiate to form viable plants.
A Problem: Somaclonal variation
Tissue culture involves successive rounds of plant division.
With increasing time in culture, the regenerated plant displays abnormalities e.g. albinism.
But What is the cause of this somaclonal variation?
Two main mechanisms are suspected..
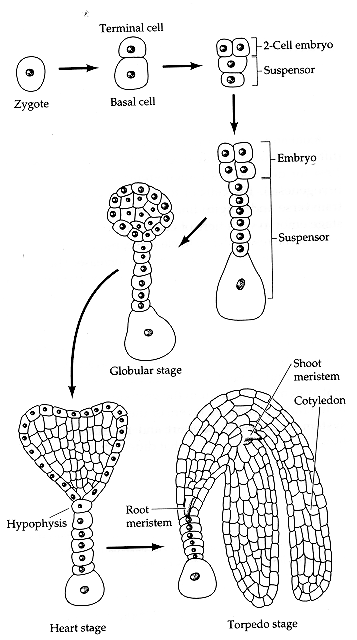
Surprisingly, certain plant tissues can be induced to form "somatic embryos" which are fundamentally
very similar to "zygotic embryos" which form within the seed.
A very attractive feature as

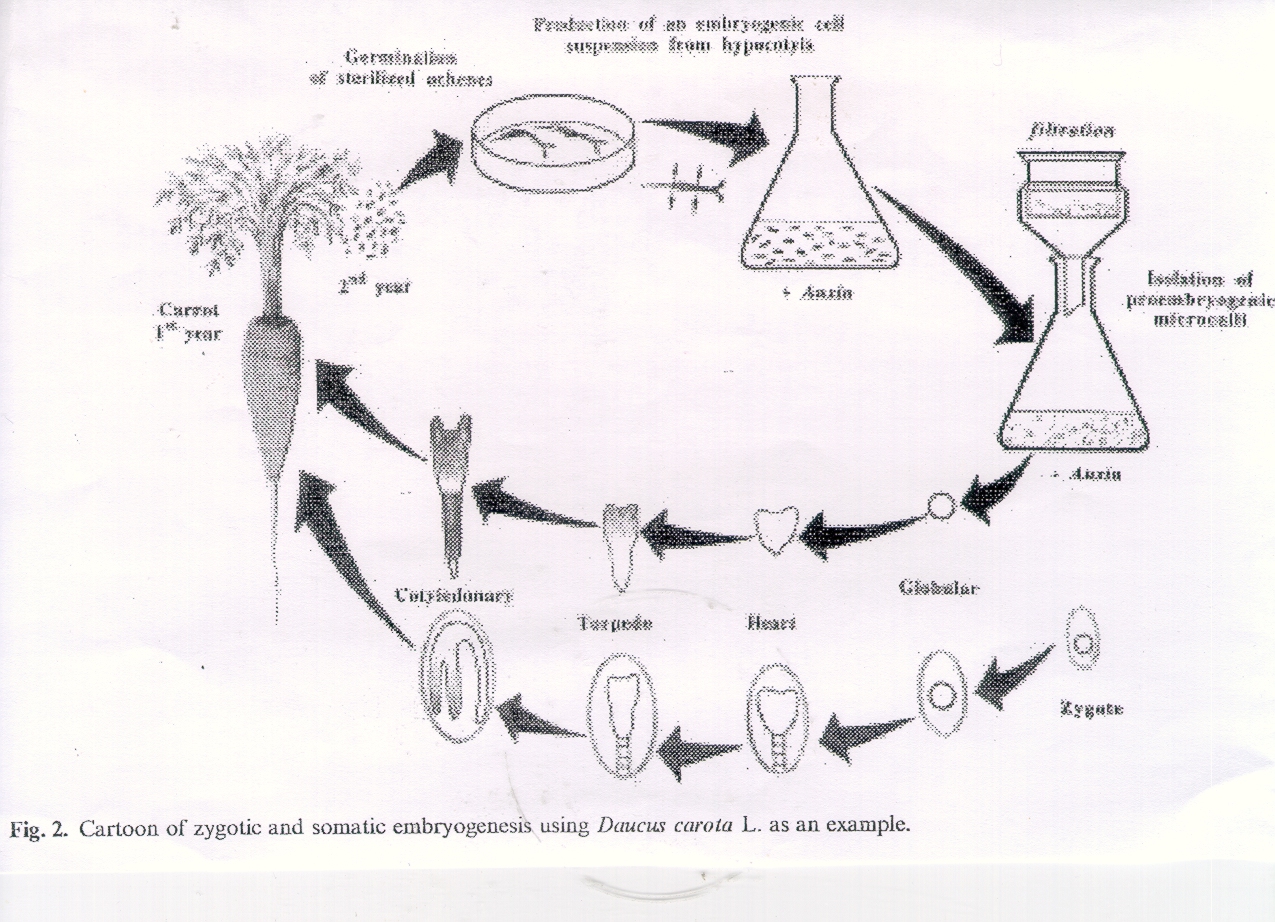
Immature embryos are a very good source of embryogenic tissue.
Embyros also form near to the surface; easy to access for transformation (see below).
Auxins: especially good at inducing embryo formation.
Culture on auxin free medium to initiate root formation. .
Certain plants are very difficult to transform using Agrobacterium. An alternative DNA involves
"bombardment " of somatic embryogenic callus with naked DNA containing the transgene of
interest and a selectable marker. This is a very inefficent process depending on the transgene
penetration of the plant nucleus and insertion into the plant genome. Nevertheless, this is the
current technique
of choice when transforming cereal plants.
The DNA coated onto tungsten / gold beads..Microcarriers
The DNA/microcarroers are accelerated on to plant/ explant/ callus samples using gunpowder,
carbon dioxide, electric charge or helium.
Using 12 separate plasmids up to 600kb DNA can be introduced at once particle bombardment.

Optimised by
1. Agrolistics : A half way house between Agrobacterium and biolistics

2. Protoplast fusion (see slide-show to see what protoplasts look like)
-
Cell wall is a significant barrier to “naked” DNA uptake.
-
Most “fussy” technique.
-
Mechanical and/or enzymatic digestion
-
Cellulase
-
Pectic lyase
-
Cocktail enzymes “Macerozyme”
-
Some cell –types are more competent for DNA uptake than other.
e.g. sugar beet; enrichment for guard cell protoplasts by differential
centrifugation.
-
DNA uptake: Involves perturbation of the plasma membrane
1. Polyethylene glycol.- amphipathic molecule which alters the mobility
of the
membrane phospholipids.
2. Electroporation – Use electric charge to “punch” hole in membrane
3. Liposomes.